Overlap of Adjacent p Orbitals-Electron Delocalization
- Page ID
- 812
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Have you ever wondered why color exists? If you know it is because of molecules, do you know what makes the different colors appear? Most of it can be explained by the presence of the π bonds, and how many there are. When photons hit the atoms and excite the electrons, the bonds that the electrons are in affect the frequency of light that is reflected when the electrons go back to th ground state and is perceived by our eyes.
Introduction
π-Bonds are formed from the overlap of two adjacent parallel p orbitals (Fig.1). π bonds are important in the addition reactions. It is vital in synthesising many products like the polymers we use in modern day society. These systems have incredibly unique thermodynamic and photochemical properties. In this section we discover the special properties that three adjacent p orbitals (a π double bond and a p orbital) can have on the reactivity of a carbon center. We call a carbon next to a double bonded carbon an allylic carbon. The electrons that are shared between all the atomic centers are commonly said to be delocalized.
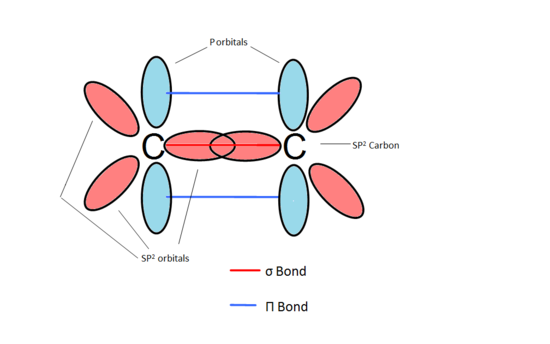
Figure 1 A π Bond is the interaction between the two p orbitals of the bonded carbons.
Allylic Carbocations
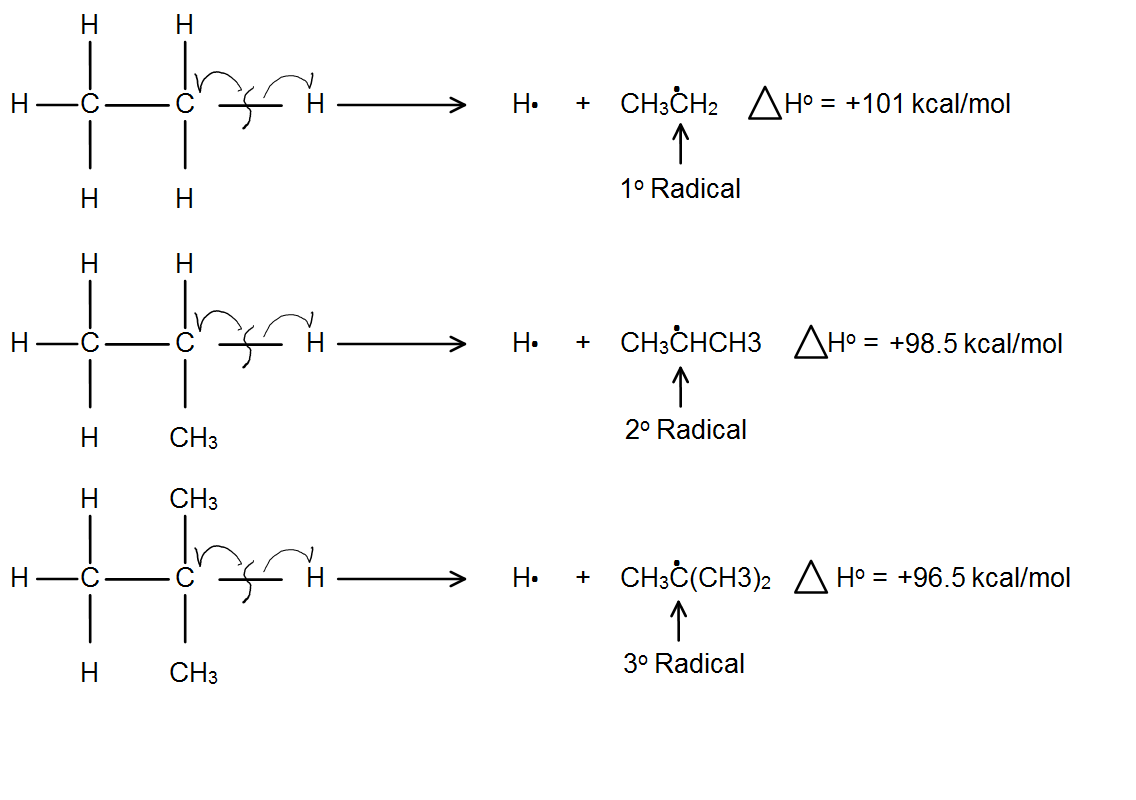
Lets take a look at the energy required to homolytically cleave (bond enthalpies) a covalent bond fig.2
.png?revision=1&size=bestfit&width=620&height=436)
Figure 2: shows the decreasing energy required to a cleave a bond as we add methyl groups (for stabilization)
As you can see, the more stable the carbocation, the easier it is to form. The tertiary carbocation takes less energy to cleave than the primary carbocation. This is due to hyperconjugation which stabilizes the free electron by the slight interaction with nearby bond orbitals in the methyl groups.
Now we look at a propene molecule Figure 3.

Figure 3: propene radical formation
Even though the carbocation is a primary one, the energy required to cleave the bond is even lower than that of a seemingly stable tertiary structure. Also, the pKa of propene is about 40, compared to propane, which is about 50. This means the propene is much more willing to form the propenyl anion, about ten orders of magnitude more. Let's take a look at why:
Resonance
One way to explain the stability is in terms of resonance, the allylic effect can be shown in all carbon center forms: Cation, Radical, and Anion in figure 4. The double bond's ability to shift in between different carbons gives it extra resonance forms, which lends extra stability.
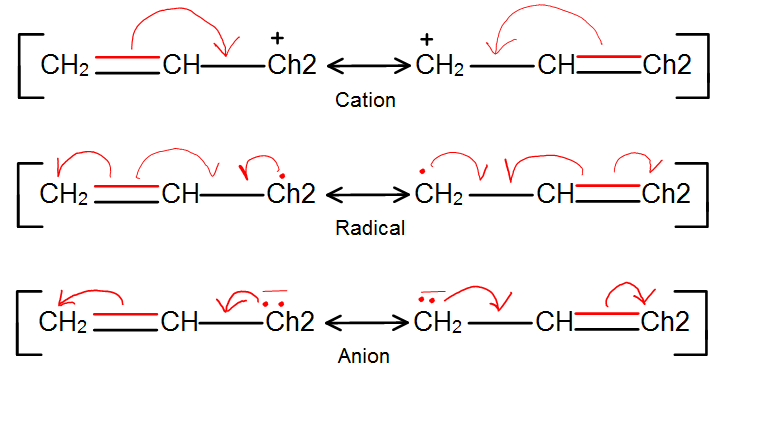
Figure 4: Each type of center is stabilized through resonance because double bond
Molecular Orbitals
It can also be clearly shown through orbitals in figure 5.

Figure 5. A conjugated ? electron system
The stability of the carbocation of propene is due to a conjugated π electron system. A "double bond" doesn't really exist. Instead, it is a group of 3 adjacent, overlapping, non-hybridized p orbitals we call a conjugated π electron system. You can clearly see the interactions between all three of the p orbitals from the three carbons resulting in a really stable cation. It all comes down to where the location of the electron-deficient carbon is.
Molecular orbital descriptions can explain allylic stability in yet another way using 2-propenyl. Fig.6
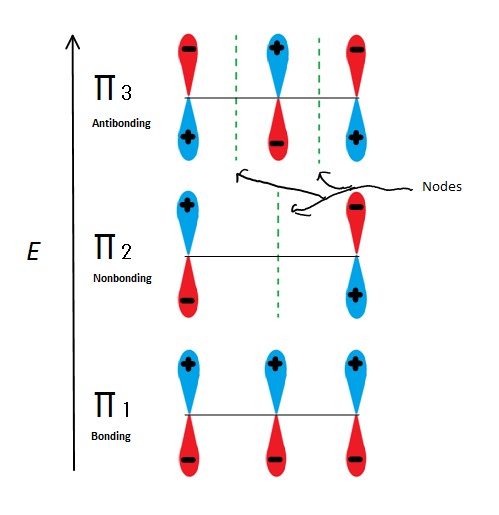
Figure 6: Shows the 3 possible Molecular orbitals of 2-propenyl
If we just take the π molecular orbital and not any of the s, we get three of them. π1 is bonding with no nodes, π2 is nonbonding (In other words, the same energy as a regular p-orbital) with a node, and π3 is antibonding with 2 nodes (none of the orbitals are interacting). The first two electrons will go into the π1 molecular orbital, regardless of whether it is a cation, radical, or anion. If it is a radical or anion, the next electron goes into the π2 molecular orbital. The last anion electron goes into the nonbonding orbital also. So no matter what kind of carbon center exists, no electron will ever go into the antibonding orbital.
The Bonding orbitals are the lowest energy orbitals and are favorable, which is why they are filled first. Even though the nonbonding orbitals can be filled, the overall energy of the system is still lower and more stable due to the filled bonding molecular orbitals.
This figure also shows that π2 is the only molecular orbital where the electrion differs, and it is also where a single node passes through the middle. Because of this, the charges of the molecule are mainly on the two terminal carbons and not the middle carbon.
This molecular orbital description can also illustrate the stability of allylic carbon centers in figure 7.
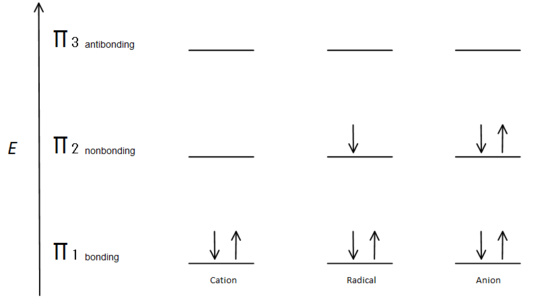
Fig.7 diagram showing how the electrons fill based on the Aufbau principle.
The π bonding orbital is lower in energy than the nonbonding p orbital. Since every carbon center shown has two electrons in the lower energy, bonding π orbitals, the energy of each system is lowered overall (and thus more stable), regardless of cation, radical, or anion.
Ultraviolet and Visible Spectroscopy
An electronic spectra can indicate the relative amounts of delocalization in a π electron system. The more conjugated π bonds there are, the longer the wavelength is reflected. Ethene has a maximum wavelength of 171 nm compared to 1,4-Pentadiene, which has a maximum wavelength of 178 nm and 1,3-Butadiene which has a maximum wavelength of 217 nm. The higher wavelength of the 1,3-Butadiene compared to only 178 nm for 1,4-Pentadiene shows the unique difference between a conjugated an non conjugated system.
References
- Kent, Doug. 2009. Lecture on February 4, General assistance in Chem 118B workshop. University of California, Davis.
- Loudon, G. Marc. Organic Chemistry. 4th ed. New York: Oxford University Press, 2002.
- Vollhardt, Peter, and Neil Shore. Organic Chemistry: Structure and Function. 5th. New York: W.H. Freeman and Company, 2007.
Problems
- Given 1-bromopropane and water, or 1-bromopropene, which one would react and why?
- Order these in terms of free radical stability, 1 being most stable: tertiary alkyl, primary alkyl, secondary allylic, tertiary allylic, .CH3, primary allylic, secondary alkyl.
- Order these molecules in terms of relative wavelength, from longest, to shortest.
- 1,3-Cyclopentene; 2-Methyl-1,3-Pentadiene; Pyrrole; 2-Methyl-1,4-Pentadiene; trans-1,3,5-Hexatriene.
- Draw out the resonance structures of the arenium ion during a general electrophilic aromatic substitution of a Benzene ring.
Answers
- 1-bromopropane would have no reaction because water is a poor nucleophile, and a primary carbocation would form. 1-bromopropene is allylic and so stabilizes the carbon attached to the bromine. The water can then successfully react with it through an SN2 reaction.
- 1 tertiary allylic, 2 secondary allylic~tertiary alkyl, 3 primary allylic~secondary alkyl, 4 primary alkyl, 5 .CH3
- Long wavelength Pyrrole>1,3-Cyclopentadiene>trans-1,3,5-Hexatriene>2-Methyl-1,3-Pentadiene>2-Methyl-1,4-Pentadiene short wavelength
Contributors
- Jeffrey Hu

