Carboxylic Derivatives - Synthesis Applications
- Page ID
- 1221
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Applications of Condensation Reactions to Synthesis
The construction of complex molecules by a series of suitable reactions carried out from simple starting compounds is called synthesis. Synthesis is not only of immense practical importance (aspirin and nylon are two examples of commercially valuable synthetic compounds), but it also allows us to prepare novel molecules with which to test our understanding of structure and reactivity. Three challenges must be met in devising a synthesis for a specific compound:
- The carbon atom framework or skeleton that is found in the desired compound (the target) must be assembled.
- The functional groups that characterize the target compound must be introduced or transformed from other groups at appropriate locations.
- If centers of stereoisomerism are present, they must be fixed in a proper manner.
Recognition of these tasks does not imply that they are independent of each other, or should be approached and solved separately. A successful plan or strategy for a synthesis must correlate each step with all these goals, so that an efficient and practical solution to making the target molecule is achieved. Nevertheless, it is useful to classify the various reactions we have studied with respect to their ability to (i) enlarge or expand a given structure, (ii) transform or relocate existing functional groups, and (iii) do both of these in a stereoselective fashion. The organization of this text by functional group behavior partially satisfies the second point, and the following discussion focuses on the first.
1. Carbon-Carbon Bond Formation
A useful assortment of carbon-carbon bond forming reactions have been described in this and earlier chapters. These include:
![]() (1) Friedel-Crafts alkylation and acylation.
(1) Friedel-Crafts alkylation and acylation.
![]() (2) Diels-Alder cycloaddition.
(2) Diels-Alder cycloaddition.
![]() (3) addition of organometallic reagents to aldehydes, ketones & carboxylic acid derivatives.
(3) addition of organometallic reagents to aldehydes, ketones & carboxylic acid derivatives.
![]() (4) alkylation of acetylide anions.
(4) alkylation of acetylide anions.
![]() (5) alkylation of enolate anions.
(5) alkylation of enolate anions.
![]() (6) Claisen and aldol condensations.
(6) Claisen and aldol condensations.
With the exception of Friedel-Crafts alkylation these reactions all give products having one or more functional groups at or adjacent to the bonding sites. As a result, subsequent functional group introduction or modification may be carried out in a relatively straightforward manner. This will be illustrated for aldol and Claisen condensations in the following section.
2. Modification of Condensation Products
A. Reactions of Aldol Products
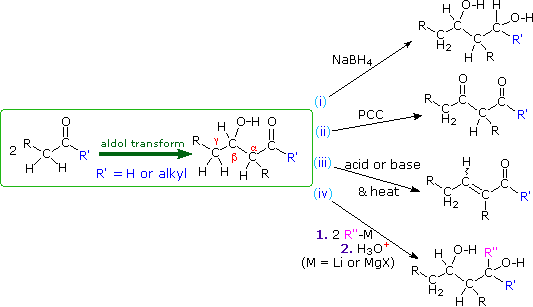
The aldol reaction produces beta-hydroxyaldehydes or ketones, and a number of subsequent reactions may be carried out with these products. As shown in the following diagram, they may be (i) reduced to 1,3-diols, (ii) a 2º-hydroxyl group may be oxidized to a carbonyl group, (iii) acid or base catalyzed beta-dehydration may produce an unsaturated aldehyde or ketone, and (iv) organometallic reagents may be added to the carbonyl group (assuming the hydroxyl group is protected as an ether or a second equivalent of reagent is used).
B. Reactions of Claisen Products
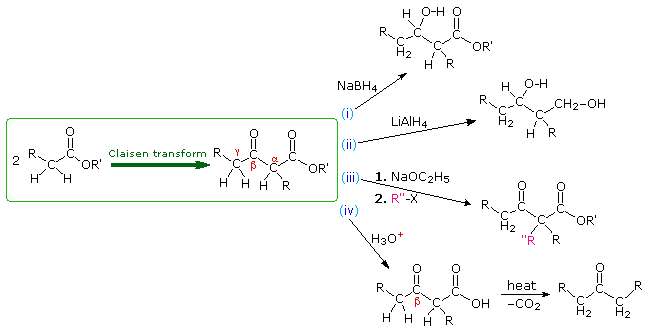
The Claisen condensation produces beta-ketoesters. These products may then be modified or enhanced by further reactions. Among these, the following diagram illustrates (i) partial reduction of the ketone with NaBH4, (ii) complete reduction to a 1,3-diol by LiAlH4, (iii) enolate anion alkylation, and (iv) ester hydrolysis followed by thermal decarboxylation of the resulting beta-ketoacid.
C. Synthesis Examples
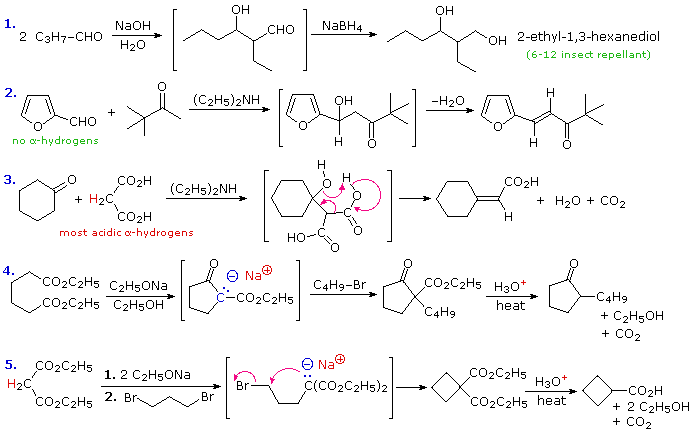
To illustrate how the reaction sequences described above may be used to prepare a variety of different compounds, five examples are provided here. The first is a typical aldol reaction followed by reduction to a 1,3-diol (2-ethyl-1,3-hexanediol). In the second example, the absence of alpha-hydrogens on the aldehyde favors the mixed condensation, and conjugation of the double bond facilitates dehydration. The doubly-activated methylene group of malonic and acetoacetic acids or esters makes them good donors in any condensation, as is demonstrated by the third aldol-like reaction. A concerted dehydrative-decarboxylation (shown by the magenta arrows) leads to the unsaturated carboxylic acid product. Amine bases are often used as catalysts for aldol reactions, as in equations #2 & 3. The fourth reaction demonstrates that the conjugate base of the beta-ketoester products from Claisen or Dieckmann condensation may be alkylated directly. Thermal decarboxylation of the resulting beta-ketoacid gives a mono-alkylated cyclic ketone. Finally, both acidic methylene hydrogens in malonic ester or ethyl acetoacetate may be substituted, and the irreversible nature of such alkylations permits strained rings to be formed. In this case thermal decarboxylation of a substituted malonic acid generates a carboxylic acid. In all these examples the remaining functional groups could be used for additional synthetic operations.
|
Vinylagous Reactions |
Some Exercises
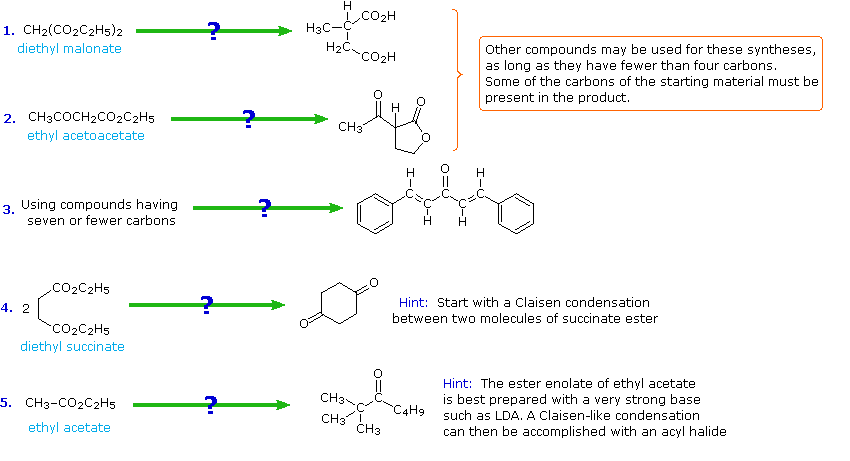
If you understand the previous discussion of reactions useful in synthesis you should try the following problems. Some of them are complex so don't be concerned if you don't solve them all immediately. Analyze each problem carefully, and try to learn from it. The solutions will be displayed by clicking the answer button under the diagram.
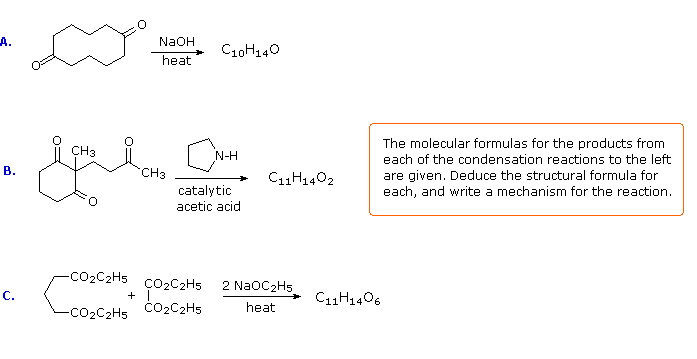
The following problems ask you to devise a synthesis for a given target molecule. The first two problems make use of the common starting materials, diethyl malonate and ethyl acetoacetate. The third problem leaves the choice of materials open. The nature of the target molecule suggests that an aldol condensation might be useful. The fourth problem must be solved by using diethyl succinate as the only reagent. Finally, other reactants composed of no more than five carbon atoms may be used in the last problem.
Contributors
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry


