Halogenation of Benzene and Methylbenzene
- Page ID
- 3978
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)This page looks at the reactions of benzene and methylbenzene (toluene) with chlorine and bromine under various conditions.
The halogenation of benzene
Substitution reactions
Benzene reacts with chlorine or bromine in the presence of a catalyst, replacing one of the hydrogen atoms on the ring by a chlorine or bromine atom. The reactions happen at room temperature. The catalyst is either aluminum chloride (or aluminum bromide if you are reacting benzene with bromine) or iron.
Strictly speaking iron is not a catalyst, because it gets permanently changed during the reaction. It reacts with some of the chlorine or bromine to form iron(III) chloride, \(FeCl_3\), or iron(III) bromide, \(FeBr_3\).
\[ 2Fe + 3Cl_2 \rightarrow 2FeCl_3\]
\[ 2Fe + 3Br_2 \rightarrow 2FeBr_3\]
These compounds act as the catalyst and behave exactly like aluminum chloride, \(AlCl_3\), or aluminum bromide, \(AlBr_3\), in these reactions.
The reaction with chlorine
The reaction between benzene and chlorine in the presence of either aluminum chloride or iron gives chlorobenzene.
![]()
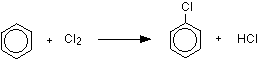
or, written more compactly:
\[ C_6H_6 + Cl_2 \rightarrow C_6H_5Cl + HCl\]
The reaction with bromine
The reaction between benzene and bromine in the presence of either aluminum bromide or iron gives bromobenzene. Iron is usually used because it is cheaper and is more readily available.
![]()
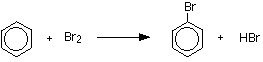
or
\[C_6H_6 + Br_2 \rightarrow C_6H_5Br + HBr\]
Addition reactions
In the presence of ultraviolet light (but without a catalyst present), hot benzene will also undergo an addition reaction with chlorine or bromine. The ring delocalization is permanently broken and a chlorine or bromine atom adds on to each carbon atom.
For example, if you bubble chlorine gas through hot benzene exposed to UV light for an hour, you get 1,2,3,4,5,6-hexachlorocyclohexane.
![]()
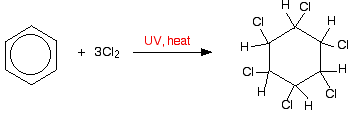
Bromine would behave similarly.
The chlorines and hydrogens can stick up and down at random above and below the ring and this leads to a number of geometric isomers. Although there aren't any carbon-carbon double bonds, the bonds are still "locked" and unable to rotate. One of these isomers was once commonly used as an insecticide known variously as BHC, HCH and Gammexane. This is one of the "chlorinated hydrocarbons" which caused so much environmental harm.
The halogenation of methylbenzene
Substitution reactions
It is possible to get two quite different substitution reactions between methylbenzene and chlorine or bromine depending on the conditions used. The chlorine or bromine can substitute into the ring or into the methyl group.
Substitution into the ring
Substitution in the ring happens in the presence of aluminum chloride (or aluminum bromide if you are using bromine) or iron, and in the absence of UV light. The reactions happen at room temperature. This is exactly the same as the reaction with benzene, except that you have to worry about where the halogen atom attaches to the ring relative to the position of the methyl group.
Methyl groups are 2,4-directing, which means that incoming groups will tend to go into the 2 or 4 positions on the ring - assuming the methyl group is in the 1 position. In other words, the new group will attach to the ring next door to the methyl group or opposite it. With chlorine, substitution into the ring gives a mixture of 2-chloromethylbenzene and 4-chloromethylbenzene.
![]()
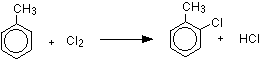
![]()
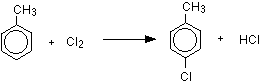
With bromine, you would get the equivalent bromine compounds.
Substitution into the methyl group
If chlorine or bromine react with boiling methylbenzene in the absence of a catalyst but in the presence of UV light, substitution happens in the methyl group rather than the ring. For example, with chlorine (bromine would be similar):
![]()
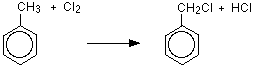
The organic product is (chloromethyl)benzene. The brackets in the name emphasize that the chlorine is part of the attached methyl group, and isn't on the ring.
One of the hydrogen atoms in the methyl group has been replaced by a chlorine atom. However, the reaction doesn't stop there, and all three hydrogens in the methyl group can in turn be replaced by chlorine atoms. That means that you could also get (dichloromethyl)benzene and (trichloromethyl)benzene as the other hydrogen atoms in the methyl group are replaced one at a time.
![]()
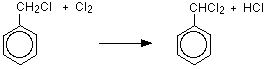
![]()
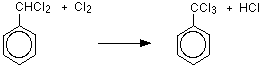
If you use enough chlorine you will eventually get (trichloromethyl)benzene, but any other proportions will always lead to a mixture of products.
Addition reactions
I haven't been able to track down anything similar to the reaction between benzene and chlorine in which six chlorine atoms add around the ring.
That perhaps isn't surprising. Chlorine adds to benzene in the presence of ultraviolet light. With methylbenzene under those conditions, you get substitution in the methyl group. That is energetically easier because it doesn't involve breaking the delocalized electron system.
Whether you would get addition to the ring if you used a large excess of chlorine and did the reaction for a long time, I don't know. Once all the hydrogens in the methyl group had been substituted, perhaps you might then get addition to the ring as well.
Contributors
Jim Clark (Chemguide.co.uk)


