Friedel-Crafts Reactions
- Page ID
- 3979
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)This page gives details of the Friedel-Crafts reactions of benzene and methylbenzene (toluene).
Friedel-Crafts acylation of benzene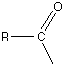
An acyl group is an alkyl group attached to a carbon-oxygen double bond. If "R" represents any alkyl group, then an acyl group has the formula RCO-. Acylation means substituting an acyl group into something - in this case, into a benzene ring.
The most commonly used acyl group is CH3CO-. This is called the ethanoyl group, and in this case the reaction is sometimes called "ethanoylation". In the example which follows we are substituting a CH3CO- group into the ring, but you could equally well use any other acyl group. The most reactive substance containing an acyl group is an acyl chloride (also known as an acid chloride). These have the general formula RCOCl. Benzene is treated with a mixture of ethanoyl chloride, CH3COCl, and aluminium chloride as the catalyst. The mixture is heated to about 60°C for about 30 minutes. A ketone called phenylethanone (old name: acetophenone) is formed.


or, if you want a more compact form:
\[ C_6H_6 + CH_3COCl \longrightarrow C_6H_5COCH_3 + HCl\]
The aluminium chloride isn't written into these equations because it is acting as a catalyst. If you wanted to include it, you could write AlCl3 over the top of the arrow (see below).
Friedel-Crafts acylation of methylbenzene (toluene)
The reaction is just the same with methylbenzene except that you have to worry about where the acyl group attaches to the ring relative to the methyl group. Normally, the methyl group in methylbenzene directs new groups into the 2- and 4- positions (assuming the methyl group is in the 1- position). In acylation, though, virtually all the substitution happens in the 4- position.

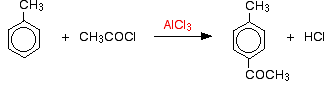
Friedel-Crafts alkylation
Alkylation means substituting an alkyl group into something - in this case into a benzene ring. A hydrogen on the ring is replaced by a group like methyl or ethyl and so on.
Friedel-Crafts alkylation of benzene: Benzene reacts at room temperature with a chloroalkane (for example, chloromethane or chloroethane) in the presence of aluminium chloride as a catalyst. On this page, we will look at substituting a methyl group, but any other alkyl group could be used in the same way. Substituting a methyl group gives methylbenzene.

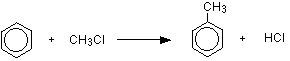
or:
\[ C_6H_6 + CH_3Cl \rightarrow C_6H_5CH_3 + HCl\]
Friedel-Crafts alkylation of methylbenzene (toluene)
Again, the reaction is just the same with methylbenzene except that you have to worry about where the alkyl group attaches to the ring relative to the methyl group. Unfortunately this time there is a problem! Where the incoming alkyl group ends up depends to a large extent on the temperature of the reaction.
At 0°C, substituting methyl groups into methylbenzene, you get a mixture of the 2-.3- and 4- isomers in the proportion 54% / 17% / 29%. That's a higher proportion of the 3- isomer than you might expect. At 25°C, the proportions change to 3% / 69% / 28%. In other words the proportion of the 3- isomer has increased even more. Raise the temperature some more and the trend continues.
Friedel-Crafts alkylation industrially
The manufacture of ethylbenzene: Ethylbenzene is an important industrial chemical used to make styrene (phenylethene), which in turn is used to make polystyrene - poly(phenylethene). It is manufactured from benzene and ethene. There are several ways of doing this, some of which use a variation on Friedel-Crafts alkylation.
The reaction is done in the liquid state. Ethene is passed through a liquid mixture of benzene, aluminium chloride and a catalyst promoter which might be chloroethane or hydrogen chloride. We are going to assume it is HCl. Promoters are used to make catalysts work better.
There are two variants on the process. One (the Union Carbide / Badger process) uses a temperature no higher than 130°C and a pressure just high enough to keep everything liquid. The other (the Monsanto process) uses a slightly higher temperature of 160°C which needs less catalyst. (Presumably - although I haven't been able to confirm this - the pressure would also need to be higher to keep everything liquid at the higher temperature.)

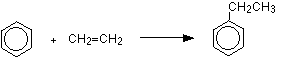
or
\[C_6H_6 + \ce{CH_2=CH_2} \rightarrow C_6H_5CH_2CH_3\]
Again, the aluminium chloride and HCl aren't written into these equations because they are acting as catalysts. If you wanted to include them, you could write AlCl3 and HCl over the top of the arrow.
Contributors
Jim Clark (Chemguide.co.uk)


