Reactions of alcohols with hydrohalic acids (HX)
- Page ID
- 15409
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)When alcohols react with a hydrogen halide, a substitution occurs, producing an alkyl halide and water:
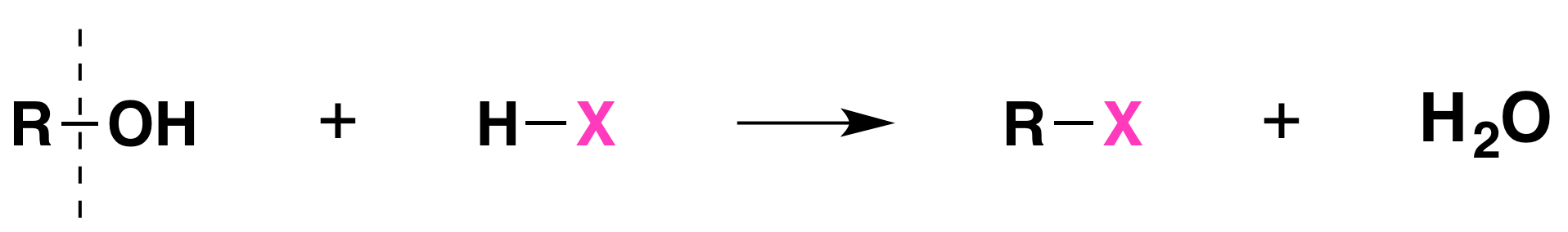
Scope of Reaction
- The order of reactivity of alcohols is 3° > 2° > 1° methyl.
- The order of reactivity of the hydrogen halides is HI > HBr > HCl (HF is generally unreactive).
The reaction is acid catalyzed. Alcohols react with the strongly acidic hydrogen halides HCl, HBr, and HI, but they do not react with nonacidic NaCl, NaBr, or NaI. Primary and secondary alcohols can be converted to alkyl chlorides and bromides by allowing them to react with a mixture of a sodium halide and sulfuric acid:
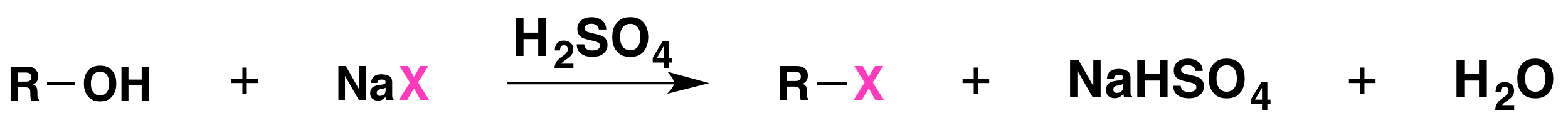
Mechanisms of the Reactions of Alcohols with HX
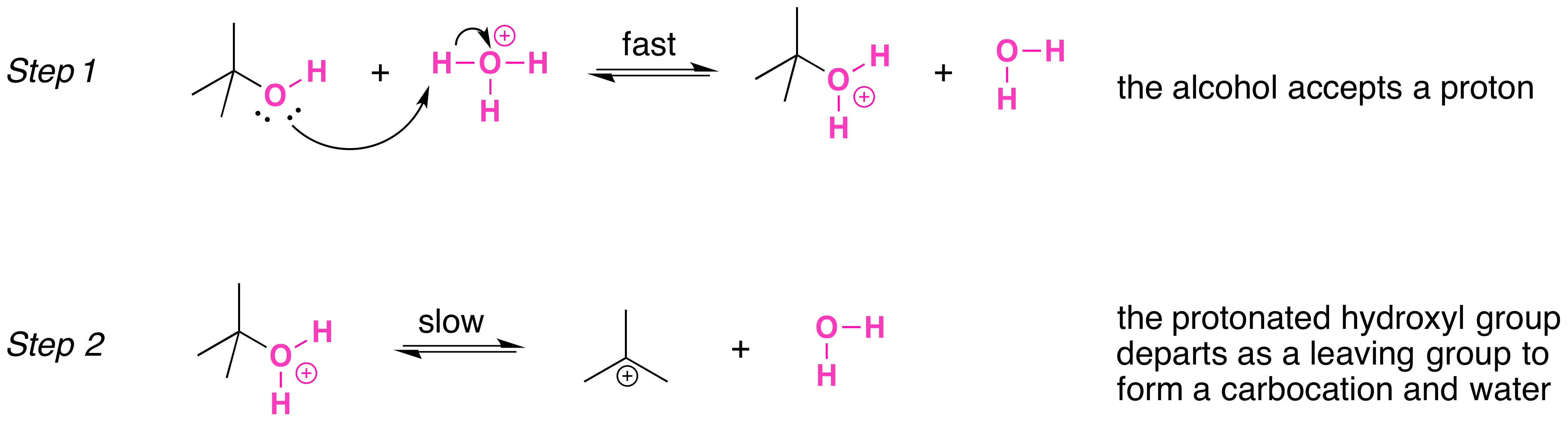
Secondary, tertiary, allylic, and benzylic alcohols appear to react by a mechanism that involves the formation of a carbocation in an \(S_N1\) reaction with the protonated alcohol acting as the substrate.
The \(S_N1\) mechanism is illustrated by the reaction tert-butyl alcohol and aqueous hydrochloric acid (\(H_3O^+\), \(Cl^-\) ). The first two steps in this \(S_n1\) substitution mechanism are protonation of the alcohol to form an oxonium ion. Although the oxonium ion is formed by protonation of the alcohol, it can also be viewed as a Lewis acid-base complex between the cation (\(R^+\)) and \(H_2O\). Protonation of the alcohol converts a poor leaving group (OH-) to a good leaving group (\)H_2O\_), which makes the dissociation step of the \(S_N1\) mechanism more favorable.
In step 3, the carbocation reacts with a nucleophile (a halide ion) to complete the substitution.

When we convert an alcohol to an alkyl halide, we perform the reaction in the presence of acid and in the presence of halide ions and not at elevated temperatures. Halide ions are good nucleophiles (they are much stronger nucleophiles than water), and because halide ions are present in a high concentration, most of the carbocations react with an electron pair of a halide ion to form a more stable species, the alkyl halide product. The overall result is an \(S_n1\) reaction.
Primary Alcohols
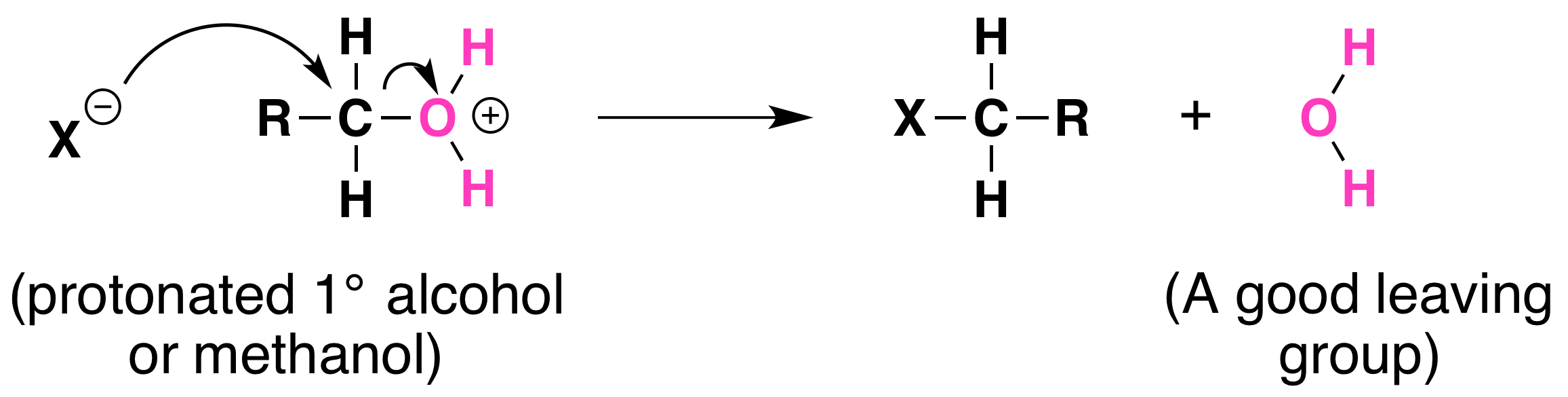
Not all acid-catalyzed conversions of alcohols to alkyl halides proceed through the formation of carbocations. Primary alcohols and methanol react to form alkyl halides under acidic conditions by an SN2 mechanism.
In these reactions, the function of the acid is to produce a protonated alcohol. The halide ion then displaces a molecule of water (a good leaving group) from carbon; this produces an alkyl halide:
Again, acid is required. Although halide ions (particularly iodide and bromide ions) are strong nucleophiles, they are not strong enough to carry out substitution reactions with alcohols themselves. Direct displacement of the hydroxyl group does not occur because the leaving group would have to be a strongly basic hydroxide ion:
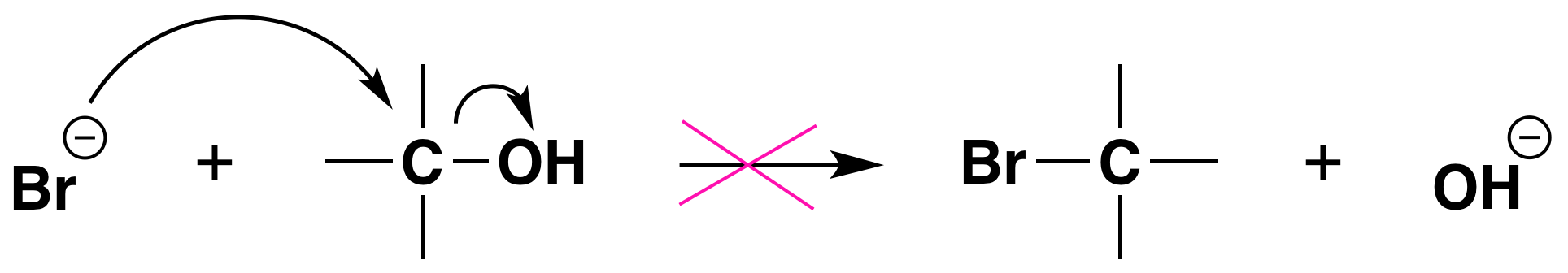
We can see now why the reactions of alcohols with hydrogen halides are acid-promoted.
The role of acid catalysis
Acid protonates the alcohol hydroxyl group, making it a good leaving group. However, other strong Lewis acids can be used instead of hydrohalic acids. Because the chloride ion is a weaker nucleophile than bromide or iodide ions, hydrogen chloride does not react with primary or secondary alcohols unless zinc chloride or a similar Lewis acid is added to the reaction mixture as well. Zinc chloride, a good Lewis acid, forms a complex with the alcohol through association with an unshared pair of electrons on the oxygen atom. This enhances the hydroxyl’s leaving group potential sufficiently so that chloride can displace it.
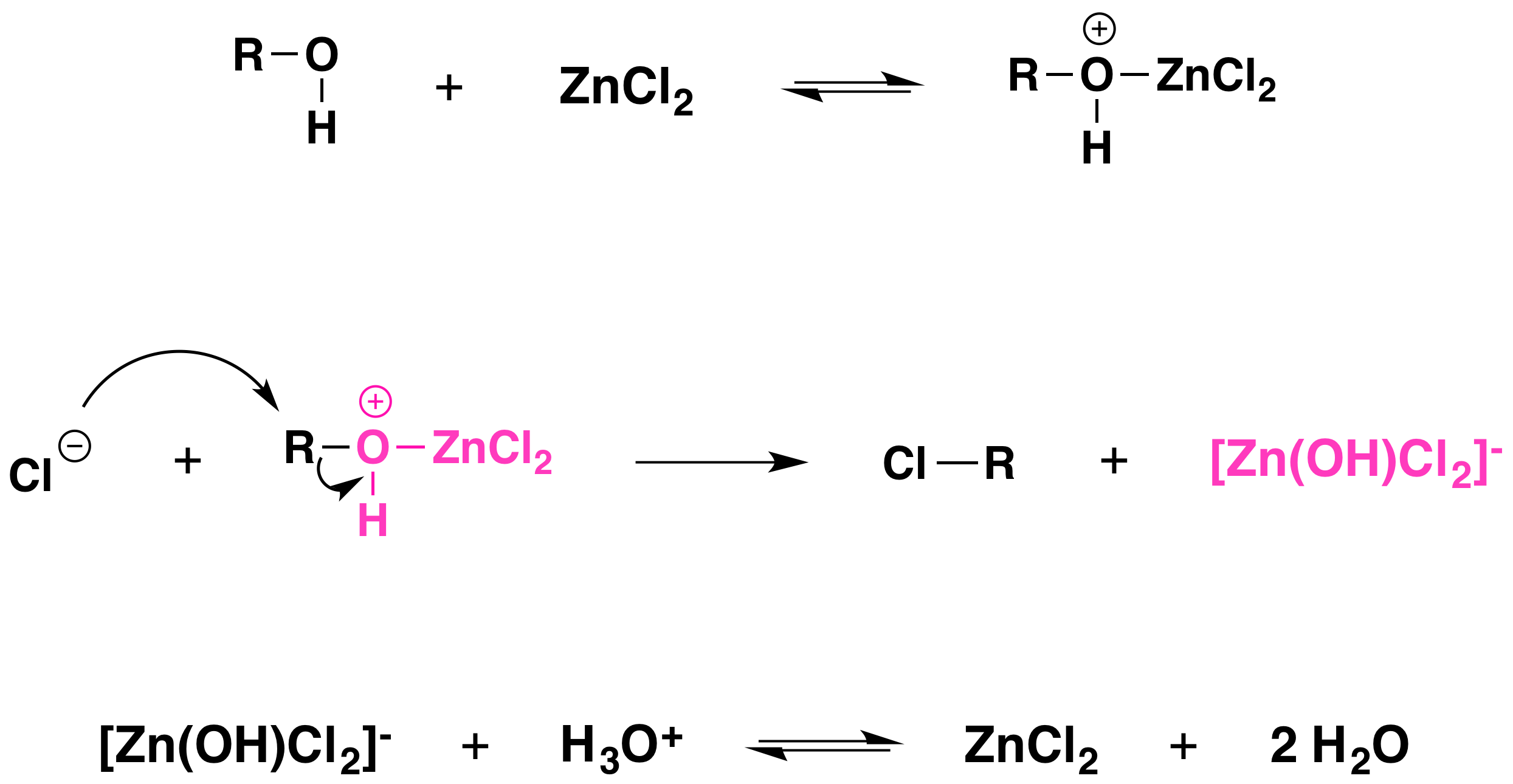
Rearrangement
As we might expect, many reactions of alcohols with hydrogen halides, particularly those in which carbocations are formed, are accompanied by rearrangements. The general rule is that if rearrangement CAN OCCUR (to form more stable or equally stable cations), it will! In these reactions, mixtures of products can be formed.

