Background
- Page ID
- 3899
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)This page defines an alcohol, and explains the differences between primary, secondary and tertiary alcohols. It examines in some detail their simple physical properties such as solubility and boiling points. Alcohols are compounds in which one or more hydrogen atoms in an alkane have been replaced by an -OH group. Alcohols fall into different classes depending on how the -OH group is positioned on the chain of carbon atoms. There are some chemical differences between the various types.
Primary alcohols
In a primary (1°) alcohol, the carbon atom that carries the -OH group is only attached to one alkyl group. Some examples of primary alcohols are shown below:
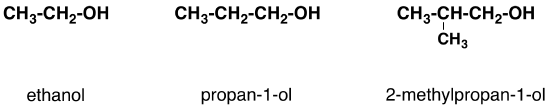
Notice that the complexity of the attached alkyl group is irrelevant. In each case there is only one linkage to an alkyl group from the CH2 group holding the -OH group. There is an exception to this. Methanol, CH3OH, is counted as a primary alcohol even though there are no alkyl groups attached to the the -OH carbon atom.
Secondary alcohols
In a secondary (2°) alcohol, the carbon atom with the -OH group attached is joined directly to two alkyl groups, which may be the same or different. Examples include the following:
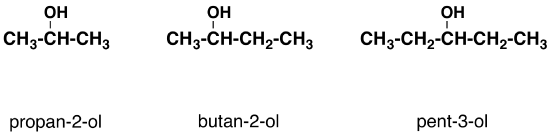
Tertiary alcohols
In a tertiary (3°) alcohol, the carbon atom holding the -OH group is attached directly to three alkyl groups, which may be any combination of the same or different groups. Examples of tertiary alcohols are given below:
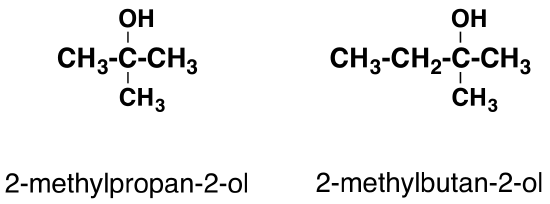
Physical properties of alcohols
Boiling Points
The chart below shows the boiling points of the following simple primary alcohols with up to 4 carbon atoms:
These boiling points are compared with those of the equivalent alkanes (methane to butane) with the same number of carbon atoms.
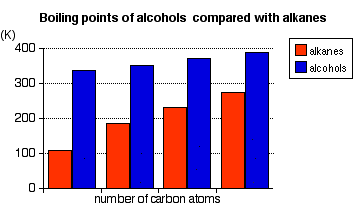
Notice that:
- The boiling point of an alcohol is always significantly higher than that of the analogous alkane.
- The boiling points of the alcohols increase as the number of carbon atoms increases.
The patterns in boiling point reflect the patterns in intermolecular attractions.
Hydrogen bonding
Hydrogen bonding occurs between molecules in which a hydrogen atom is attached to a strongly electronegative element: fluorine, oxygen or nitrogen. In the case of alcohols, hydrogen bonds occur between the partially-positive hydrogen atoms and lone pairs on oxygen atoms of other molecules.
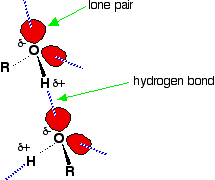
The hydrogen atoms are slightly positive because the bonding electrons are pulled toward the very electronegative oxygen atoms. In alkanes, the only intermolecular forces are van der Waals dispersion forces. Hydrogen bonds are much stronger than these; therefore, more energy is required to separate alcohol molecules than to separate alkane molecules. This is the main reason for higher boiling points in alcohols.
| Compound | IUPAC Name | Common Name | Melting Poing (oC) | Boiling Point (oC) | Solubility in H2O at 23oC |
|---|---|---|---|---|---|
| CH3OH | Methanol | Methyl alcohol | -97.8 | 65.0 | Infinite |
| CH3Cl | Chloromethane | Methyl chloride | -97.7 | -24.2 | 0.74 g/100 mL |
| CH4 | Methane | -182.5 | -161.7 | 3.5 mL (gas)/ 100 mL | |
| CH3CH2OH | Ethanol | Ethyl alcohol | -114.7 | 78.5 | Infinite |
| CH3CH2Cl | Chloroethane | Ethyl chloride | -136.4 | 12.3 | 0.447 g/100 mL |
| CH3CH3 | Ethane | -183.3 | -88.6 | 4.7 mL (gas)/ 100 mL | |
| CH3CH2CH2OH | 1-Propanol | Propyl alcohol | -126.5 | 97.4 | Infinite |
| CH3CH2CH3 | Propane | -187.7 | -42.1 | 6.5 mL (gas)/ 100 mL | |
| CH3CH2CH2CH2OH | 1-Butanol | Butyl alcohol | -89.5 | 117.3 | 8.0 g/100 mL |
| CH3(CH2)4OH | 1-Pentanol | Pentyl alcohol | -79 | 138 | 2.2 g/100 mL |
This table shows that alcohols (in red) have higher boiling points and greater solubility in H2O than haloalkanes and alkanes with the same number of carbons. It also shows that the boiling point of alcohols increase with the number of carbon atoms.
The effect of van der Waals forces
- Boiling points of alcohols: Hydrogen bonding is not the only intermolecular force alcohols experience. They also experience van der Waals dispersion forces and dipole-dipole interactions. The hydrogen bonding and dipole-dipole interactions are similar for all alcohols, but dispersion forces increase as the size of the alcohols increase. These attractions become stronger as the molecules lengthen and contain more electrons. This increases the sizes of the temporary dipoles formed. This is why the boiling points increase as the number of carbon atoms in the chains increases. It takes more energy to overcome the dispersion forces; thus, the boiling points rise.
- Comparison between alkanes and alcohols: Even without any hydrogen bonding or dipole-dipole interactions, the boiling point of the alcohol would be higher than the corresponding alkane with the same number of carbon atoms.
Compare ethane and ethanol:
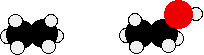
Ethanol is a longer molecule, and the oxygen atom brings with it an extra 8 electrons. Both of these increase the size of the van der Waals dispersion forces, and subsequently the boiling point. A more accurate measurement of the effect of the hydrogen bonding on boiling point would be a comparison of ethanol with propane rather than ethane. The lengths of the two molecules are more similar, and the number of electrons is exactly the same.
Solubility of alcohols in water
Small alcohols are completely soluble in water; mixing the two in any proportion generates a single solution. However, solubility decreases as the length of the hydrocarbon chain in the alcohol increases. At four carbon atoms and beyond, the decrease in solubility is noticeable; a two-layered substance may appear in a test tube when the two are mixed.
Consider ethanol as a typical small alcohol. In both pure water and pure ethanol the main intermolecular attractions are hydrogen bonds.
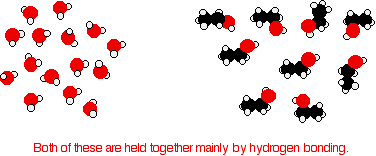
In order to mix the two, the hydrogen bonds between water molecules and the hydrogen bonds between ethanol molecules must be broken. Energy is required for both of these processes. However, when the molecules are mixed, new hydrogen bonds are formed between water molecules and ethanol molecules.
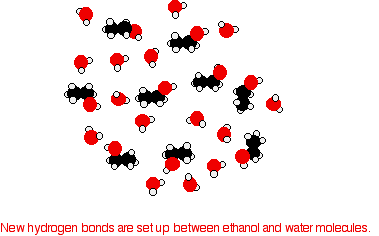
The energy released when these new hydrogen bonds form approximately compensates for the energy needed to break the original interactions. In addition, there is an increase in the disorder of the system, an increase in entropy. This is another factor in deciding whether chemical processes occur. Consider a hypothetical situation involving 5-carbon alcohol molecules.
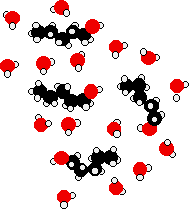
The hydrocarbon chains are forced between water molecules, breaking hydrogen bonds between those water molecules. The -OH ends of the alcohol molecules can form new hydrogen bonds with water molecules, but the hydrocarbon "tail" does not form hydrogen bonds. This means that many of the original hydrogen bonds being broken are never replaced by new ones.
In place of those original hydrogen bonds are merely van der Waals dispersion forces between the water and the hydrocarbon "tails." These attractions are much weaker, and unable to furnish enough energy to compensate for the broken hydrogen bonds. Even allowing for the increase in disorder, the process becomes less feasible. As the length of the alcohol increases, this situation becomes more pronounced, and thus the solubility decreases.
Contributors
Jim Clark (Chemguide.co.uk)


