30.1: Chain-Growth Polymers
- Page ID
- 364611
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Polymers resulting from additions to alkenes monomers are chain-growth polymers. In these processes each addition step results in a longer chain which ends in a reactive site. The mechanism of each addition step is the same, and each addition step adds another monomer to extend the chain by one repeating unit. The most common and thermodynamically favored chemical transformations of alkenes are addition reactions. Many of these addition reactions are known to proceed in a stepwise fashion by way of reactive intermediates, and this is the mechanism followed by most polymerizations. A general diagram illustrating this assembly of linear macromolecules, which supports the name chain growth polymers, is presented here. Since a pi-bond in the monomer is converted to a sigma-bond in the polymer, the polymerization reaction is usually exothermic by 8 to 20 kcal/mol. Indeed, cases of explosively uncontrolled polymerizations have been reported.
It is useful to distinguish four polymerization procedures fitting this general description.
• Radical Polymerization: The initiator is a radical, and the propagating site of reactivity (*) is a carbon radical.
• Cationic Polymerization: The initiator is an acid, and the propagating site of reactivity (*) is a carbocation.
• Anionic Polymerization: The initiator is a nucleophile, and the propagating site of reactivity (*) is a carbanion.
• Coordination Catalytic Polymerization: The initiator is a transition metal complex, and the propagating site of reactivity (*) is a terminal catalytic complex.
Radical Chain-Growth Polymerization
Virtually all of the monomers described above are subject to radical polymerization. Since this can be initiated by traces of oxygen or other minor impurities, pure samples of these compounds are often "stabilized" by small amounts of radical inhibitors to avoid unwanted reaction. When radical polymerization is desired, it must be started by using a radical initiator, such as a peroxide or certain azo compounds. The formulas of some common initiators, and equations showing the formation of radical species from these initiators are presented below.
By using small amounts of initiators, a wide variety of monomers can be polymerized. One example of this radical polymerization is the conversion of styrene to polystyrene, shown in the following diagram. The first two equations illustrate the initiation process, and the last two equations are examples of chain propagation. Each monomer unit adds to the growing chain in a manner that generates the most stable radical. Since carbon radicals are stabilized by substituents of many kinds, the preference for head-to-tail regioselectivity in most addition polymerizations is understandable. Because radicals are tolerant of many functional groups and solvents (including water), radical polymerizations are widely used in the chemical industry.
Each step in this polymer formation is an addition to an alkene. The mechanism is in most cases a free radical addition. In free radical reactions the pi pair of electrons separates. One of these electrons pairs with an electron from the attacking reagent to form a sigma bond with one of the alkene carbons. and the other electron remains attached to the other alkene carbon. (Curved arrows with only one "barb" on a point are used to follow the path of a single electron in the same way that "double-headed" arrows follow the path of an electron pair.) Intermediates with an unpaired electron are called free radicals, so this step can be described as adding a free radical to an alkene to lengthen the chain by two carbons and generate a new free radical. In its turn this new free radical can add to another molecule of monomer and continue the process.
In principle, once started a radical polymerization might be expected to continue unchecked, producing a few extremely long chain polymers. In practice, larger numbers of moderately sized chains are formed, indicating that chain-terminating reactions must be taking place. The most common termination processes are Radical Combination and Disproportionation. These reactions are illustrated by the following equations. The growing polymer chains are colored blue and red, and the hydrogen atom transferred in disproportionation is colored green. Note that in both types of termination two reactive radical sites are removed by simultaneous conversion to stable product(s). Since the concentration of radical species in a polymerization reaction is small relative to other reactants (e.g. monomers, solvents and terminated chains), the rate at which these radical-radical termination reactions occurs is very small, and most growing chains achieve moderate length before termination.
The relative importance of these terminations varies with the nature of the monomer undergoing polymerization. For acrylonitrile and styrene combination is the major process. However, methyl methacrylate and vinyl acetate are terminated chiefly by disproportionation.
Another reaction that diverts radical chain-growth polymerizations from producing linear macromolecules is called chain transfer. As the name implies, this reaction moves a carbon radical from one location to another by an intermolecular or intramolecular hydrogen atom transfer (colored green). These possibilities are demonstrated by the following equations.
Chain transfer reactions are especially prevalent in the high pressure radical polymerization of ethylene, which is the method used to make LDPE (low density polyethylene). The 1º-radical at the end of a growing chain is converted to a more stable 2º-radical by hydrogen atom transfer. Further polymerization at the new radical site generates a side chain radical, and this may in turn lead to creation of other side chains by chain transfer reactions. As a result, the morphology of LDPE is an amorphous network of highly branched macromolecules.
Cationic Chain-Growth Polymerization
Cationic polymerizations are typically acid-catalyzed. Electrophilic addition of H+ to a double bond forms a carbocation which is propagated by repeated reactions with addition alkene monomers. Alkene monomers bearing cation stabilizing groups, such as alkyl, phenyl or vinyl can be polymerized by cationic processes.

Polymerization of isobutylene (2-methylpropene) by traces of strong acids is an example of cationic polymerization. The initiation reagent cationic polymerization is commonly the Lewis acid, boron tribluoride (BF3), along with traces of water to form the acidic BF3OH-H+ catalyst. The polyisobutylene product is a soft rubbery solid, which is used for inner tubes. This process is similar to radical polymerization, as demonstrated by the following equations. Chain growth ceases when the terminal carbocation combines with a nucleophile or loses a proton, giving a terminal alkene (as shown here).
Anionic Chain-Growth Polymerization
Only monomers having anion stabilizing electron-withdrawing groups (EWG) substituents, such as phenyl, cyano or carbonyl can undergo anionic polymerization.

Species that have been used to initiate anionic polymerization include alkali metals, alkali amides, alkyl lithiums and various electron sources. The fundamental reaction for anion polymerization is a conjugate nucleophilic addition (Section 18-13) Treatment of a cold THF solution of styrene with 0.001 equivalents of n-butyllithium causes an immediate polymerization. This is an example of anionic polymerization, the course of which is described by the following equations. Chain growth may be terminated by water or carbon dioxide, and chain transfer seldom occurs.
A practical application of anionic polymerization occurs in the use of superglue. This material is methyl 2-cyanoacrylate, CH2=C(CN)CO2CH3. When exposed to water, amines or other nucleophiles, a rapid polymerization of this monomer takes place. Because the monomer has two electron withdrawing groups the polymerization particularly rapid.

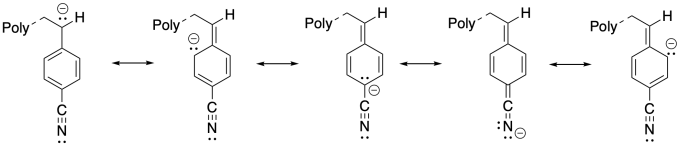
1) Anionic polymerization of p-substituted styrene proceeds very well when the substituent is an electron-withdrawing group such as nitrile. Explain the reason for the success of this approach.

2) In each group, select the alkene monomer most suitable for cationic polymerization.
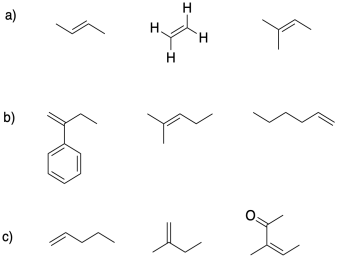
3) Provide a mechanism for the formation of a protic initiator from the interaction of boron trifluoride with water.
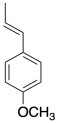
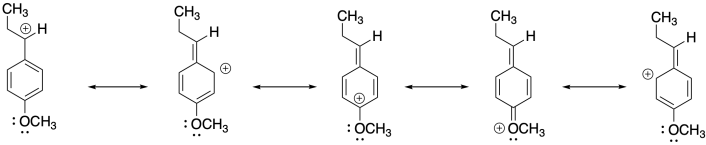
4) Anethole in a naturally-occurring compound that has been used in cationic polymerizations. Show why anethole should be a good monomer for this method.
5) Show why radicals formed from the following monomers are relatively stable:
a) acrylonitrile, CH2=CHCN
b) methyl acrylate, CH2=CHCO2Me
- Answer
-
1) The additional resonance stabilization of the anion by the nitrile group makes anionic polymerization proceed smoothly.
2)

3)

4)
5)


