23.4: Using Aldol Reactions in Synthesis
- Page ID
- 36426
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to identify the aldehyde or ketone and other necessary reagents that should be used to prepare a given enone by an aldol condensation.
This section stresses the importance of being able to think logically.
The experience that you have already gained through designing multi‑step syntheses and solving road‑map problems should help you to recognize when an aldol reaction may have been one of the steps in the synthesis of a given compound.
It is important that you recognize that the aldol condensation is an important part of a synthetic chemist’s repertoire, both because it involves the formation of a new carbon‑carbon bond, and also because it yields a product containing two functional groups.
Aldol reactions are excellent methods for the synthesis of many enones or beta-hydroxy carbonyls. Because of this, being able to predict when an aldol reaction might be used in a synthesis in an important skill. This can be accomplished by identifying these combinations of atoms and bonds and then, working backwards, theoretically breaking the target molecule apart into possible reactants.
Fragments which are easily formed by an aldol reaction
Determining the Reactants for an Aldol Condensation
During an aldol condensation a C-C sigma and a C-C pi bond are formed. This makes the key bond cleavage in the target molecule the C=C bond between the carbons alpha and beta away from the carbonyl. After the cleavage, the carbon that was in the alpha position (on the fragment with the carbonyl) gains two hydrogens. The carbon that was in the beta gains a =O to form a carbonyl.
Determining the Reactants for an Aldol Reaction
During an aldol condensation a C-C sigma bond is formed. This makes the key bond cleavage in the target molecule the C-C bond between the carbons alpha and beta away from the carbonyl. After the cleavage, the carbon that was in the alpha position (the fragment with the carbonyl) gains one hydrogen. The fragment loses a hydrogen from the OH and then forms a C=O carbonyl bond.
Worked Example
Show how the following molecule could be made using an aldol condensation?
- Answer
-
Analysis: The C=C bond in the target molecule is cleaved to form two fragments. The fragment with the carbonyl gains two alpha hydrogens. The other fragment gains a =O to form a carbonyl. Both fragments end up producing the same reactant which is typical for an aldol condensation.
Solution
Additional Synthetic Considerations
The enone product of an aldol condensation is versatile because it contains two functional groups (alkene & carbonyl) which can be subject to further reactions. Among many possible reactions, an enone can undergo hydrogenation to produce an aldehyde or ketone. Also, the carbonyl group can undergo hydride reduction to produce a beta-hydroxyalkene.
These additional reactions can be applied with the consideration of using an aldol reaction in the synthesis of a target molecule. A similar analysis can be extrapolated to the other reactions possible with the alkene and carbonyl present in an enone.
Analysis for Hydrogenation
To consider a hydrogenation, remove a hydrogen from a carbon in both the alpha and beta positions relative to the carbonyl. Then connect these two carbons with a C=C double bond. This create a possible enone which can be broken apart further using the analysis described above. Target molecules can have often have multiple alkyl chains which can be used to form a double bond.
Analysis for Hydride Reduction
To consider a hydride reduction remove a hydrogen from the alcohol oxygen then a hydrogen from the adjacent carbon. Connect the oxygen and carbon with a double bond to form a C=O carbonyl. This provides an enone which can undergo further analysis.
Please devise a synthesis pathway for the following molecule using an aldol reaction:
- Answer
-
Analysis: Because the target molecule has two beta-carbons with hydrogens there are two possible synthesis pathways. Both should be considered for their effectiveness. Because a carbonyl is already present fragmentations will first be performed to create two possible enone intermediates. These enones will both be broken into their aldol reactants. When looking a possible aldol reactants produced by each pathway it is clear that pathway 2 is preferred. Because both fragments are the same they will react to form on major product.
Solution

1) Which of the following molecules could be made using an aldol reaction or condensation. Please show the starting material for the reactions that are possible.
2) Please show the reaction steps required to make 4-methyl-2-pentanol from an aldol condensation.
3) Please design a synthesis for the following molecule: Red = Oxygen, Grey = Carbon, White = Hydrogen
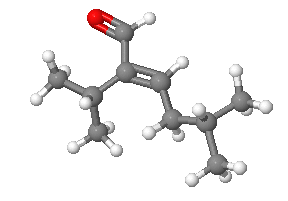
- Answers
-
1) Molecules A and C are possible.
Solution for molecule A
Solution for molecule C
2)
3)


