21.7: Chemistry of Amides
- Page ID
- 36404
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- write an equation to describe the preparation of an amide from an acid chloride.
- identify the amide linkage as the basic unit from which all proteins are made, and hence recognize the importance of the amide linkage to biologists and biochemists.
- write detailed mechanisms for the acidic and basic hydrolysis of amides.
- write an equation to describe the reduction of an amide to an amine.
- write a detailed mechanism for the reduction of an amide to an amine.
- identify the product formed when a given amide is reduced with lithium aluminum hydride.
- identify the amide, the reagents, or both, necessary to prepare a given amine by direct reduction.
- identify lactams as being cyclic amides which undergo hydrolysis and reduction in a manner analogous to that of their acyclic counterparts.
Make certain that you can define, and use in context, the key term below.
- lactam
When we talk about amino acids, we are generally referring to α‑amino acids; that is, compounds in which an amino (NH2) group and a carboxyl group are attached to the same carbon atom:
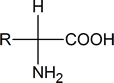
Notice that such compounds contain a chiral carbon atom (unless R = H, which is glycine). Proteins can be considered to consist of amino acid residues joined by amide (or peptide) links. These peptide links consist of exactly the same structural units that we find in N‑substituted primary amides.
A lactam is a cyclic amide, in the same way that a lactone is a cyclic ester:
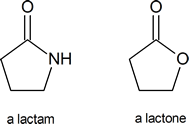
Among the most important molecules that contain lactam rings are the penicillins:
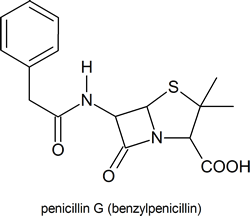
The amide functional group is extremely important for biological molecules because amides make up the backbone of proteins. Proteins are actually polymers of amino acids, linked by amide groups known as peptide bonds. Proteins (polymers of ~40 amino acids or more) and peptides (shorter polymers) are formed when the amino group of one amino acid monomer reacts with the carboxylate carbon of another amino acid to form an amide linkage, which in protein terminology is a peptide bond. The individual amino acids within a peptide or protein are called amino acid residues.
Compounds containing amides have a wide range of biological activity and include penicillin G an antibiotic, piperine which is responsible for the pungent flavor of black pepper, and anandamide which provides some of the pleasurable sensations gained from eating chocolate.
Preparation of Amides
Amides are most commonly prepared though the reaction of an acid chloride with ammonia, a 1o amine, or 2o amine. In an analogous reaction, an amide can be prepared through the reaction of a carboxylic acid and an amine using a coupling agent such as DCC. Simple amides can be prepared by reacting an acid anhydride with an amine. Lastly, amides can be formed through the direct reaction of a carboxylic acid and an amine. However, this reaction is rarely used because the conditions are relatively severe.
Reactions of Amides
Amides are relatively unreactive towards nucleophilic acyl substitutions due to the poor leaving group ability of its nitrogen containing Y group. Despite this, amides can react with water under acidic or basic conditions to create a carboxylic acid through nucleophilic acyl substitution. Reaction of primary amides with thionyl chloride (SOCl2) creates nitriles. Hydride reduction using LiAlH4 causes the carbonyl oxygen of the amide to eliminate as a leaving group creating the corresponding amine.
Lactams, cyclic amides, are affected by these reactions in the same fashion as acyclic amides.
Conversion of Amides to Carboxylic Acids: Hydrolysis
Amides can be hydrolyzed into a carboxylic acid and ammonia or an amine by heating in an acidic or basic aqueous solution. In both cases, acid-base reactions occurring with the products make the overall reaction irreversible. Under acidic conditions the amine produced by the reaction is protonated to form a non-nucleophilic ammonium compound. Under basic conditions the carboxylic acid produced by the reaction is deprotonated to a non-electrophilic carboxylate.
General Reaction
Examples
Mechanism Under Acidic Conditions
Protonation of the amide increases the electrophilicity of the carbonyl carbon allowing water to add causing formation of a tetrahedral oxonium intermediate. Next a proton is transferred to the amide nitrogen giving it a positive charge and increasing its ability to act as a leaving group. Reforming the carbonyl double bond causes the elimination of an amine as a leaving group, creating a protonated carboxylic acid. In the last step of the mechanism, the produced amine acts as a base, removing a hydrogen, to form a carboxylic acid and an ammonium compound. This final deprotonation step essentially removes the amine from the equilibrium which drives the reaction towards completion. Since the amine is no longer part of the equilibrium, the reaction is effectively irreversible.
1) Protonation of the carbonyl
2) Nucleophilic attack by water
3) Proton transfer
4) Leaving group removal
5) Deprotonation
Mechanism Under Basic Conditions
The base-promoted hydrolysis of an amide follows the typical nucleophilic acyl substitution mechanism. A full equivalent of hydroxide anion is used, so the reaction is called base-promoted and not base catalyzed. The mechanism of basic amide hydrolysis begins with the nucleophilic addition of a hydroxide ion at the carbonyl carbon creating a tetrahedral alkoxide intermediate. The carbonyl bond is reformed along with the elimination of an amide ion (-NHR) leaving group (note this is a different use of the word amide meaning an amine anion), forming a carboxylic acid. In the last step, the amide ion deprotonates the carboxylic acid to form a carboxylate salt and an amine as products. This final deprotonation step essentially removes the carboxylic acid from the equilibrium which drives the reaction towards completion. Since the carboxylic acid is no longer part of the equilibrium, the reaction is effectively irreversible.
1) Nucleophilic attack by hydroxide
2) Leaving group removal
3) Deprotonation
Hydrolysis of Proteins
Hydrolysis of amide bonds is the first step in the metabolism of dietary proteins. Protein hydrolysis is catalyzed by protease enzymes (abbreviated Asp1 and Asp2 in the figure below). The mechanisms starts with the nucleophilic acyl substitution by water while breaking the amide C-N bond. This produces a protein fragment with an amine and another with a carboxylic acid. The amine fragment will deprotonate the carboxylic acid forming the carboxylate and ammonium fragments.
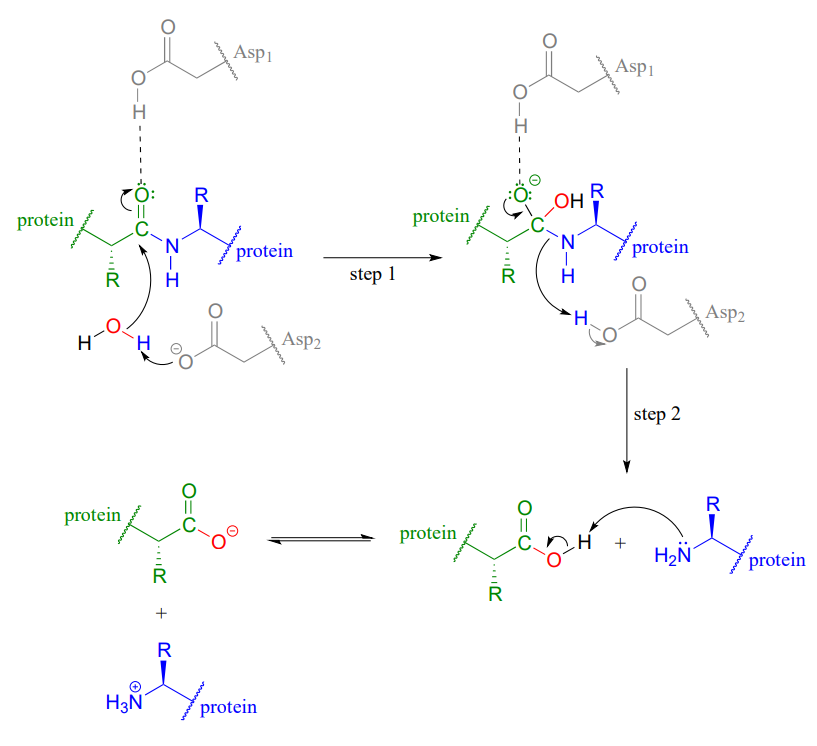
Conversion of 1o Amides to Nitriles: Dehydration
1o Amides can be converted into a nitrile through reaction with thionyl chloride (SOCl2). Sulfurdioxide (SO2) and hydrochloric acid are produced as byproducts.
General Reaction
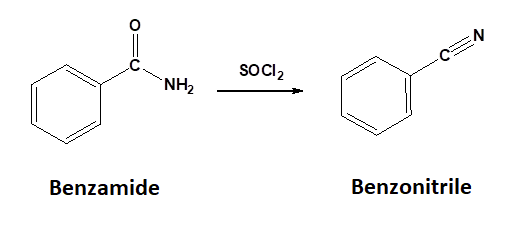
Mechanism for the Conversion of a 1o Amide to a Nitrile
During the first step of the mechanism, the amide acts as a nucleophile and attacks the electrophilic sulfur of thionyl chloride, pushing the pi electrons of the S=O bond onto oxygen. Removal of a chloride anion as a leaving group allows the sulfoxide S=O bond to reform creating a protonated imine chlorosulfite. Deprotonation forms an imine chlorosulfite intermediate. A second deprotonation eliminates the chlorosulfite leaving group as sulfur dioxide and a chloride anion and forms the C-N triple bond of the nitrile product.
1) Nucleophilic attach on thionyl chloride
2) Leaving group removal
3) Deprotonation
4) Leaving group removal
Conversion of Amides to 1o, 2o or 3o Amines: Hydride Reduction
Amides are reduced to amines by treatment with LiAlH4, and this has proven to be one of the most general methods for preparing all classes of amines (1º, 2º & 3º). Due to the nitrogen in the Y group of amides, the outcome of LiAlH4 reductions is distinctly different than for esters since amide anions are poorer leaving groups than alkoxide anions. Furthermore, oxygen forms especially strong bonds to aluminum. During this reaction the carbonyl oxygen of the amide is removed as a leaving group and not the nitrogen containing Y group. Removal of the leaving group allows for a formation of a C=N iminium bond which can accept a second addition of a hydride nucleophile to form the amine.
General Reaction
Predicting the Products of a Hydride Reduction
There are two major changes in bonding during this reaction: 1) The C=O carbonyl is removed from the starting material 2) Two hydride nucleophiles are added to what was the carbonyl carbon of the amide.
Example
Alkyl groups attached to the nitrogen do not affect the reaction.
Mechanism for the Hydride Reduction of an Amide to Form an Amine
Addition of a hydride nucleophile to the carbonyl carbon of the amide produces a tetrahedral alkoxide intermediate. A Lewis acid-base interaction occurs between the alkoxide (Lewis Base) and AlH3 (Lewis acid) forming a complex with an O-Al bond. The nitrogen lone pair forms a double bond with the previous carbonyl carbon ejecting a metal oxide species (e.g. [H3Al–O]2–) as a leaving group, and while forming an iminium double bond. Addition of a second equivalent of hydride nucleophile to the iminium carbon produces an amine.
-
1) Nucleophilic attack by the hydride
2) Complex formation
3) Leaving group removal
4) Nucleophilic attach by the hydride
How could the following molecule be synthesized using an aminolysis of an acid chloride?
- Answer
-
One of the preferred methods for making amines is through a nucleophilic acyl substitution using an acid chloride and amine to form an amide. The amide is then reduced to the amine during a hydride reduction with LiAlH4.
In the first step of retrosynthetic analysis, one of the carbons alpha to the amine nitrogen is converted to a carbonyl thus creating an amide intermediate. The key bond formed during this pathway is the C-N sigma bond between the carbonyl carbon and the nitrogen created during amide formation. Breaking this bond separated the target molecule into the two starting materials for the pathway. The carbonyl carbon gains a –Cl to become an acid chloride and the nitrogen fragment gains an H to become a 1o amine. In the forward reaction pathway, the acid chloride and the 1o amine are linked to from an amide in the first step of the reaction. Subsequent hydride reduction of the amide using LiAlH4 produces the amine target molecule.
The target molecule has two alpha carbons which could possibly be converted to a carbonyl. This allows there to be two possible synthetic pathways.
Pathway 1
Solution 1
Pathway 2
Solution 2
1) How would you prepare the following compounds from N-Propypl benzamide?
(a)
(b)
(c)
2) Propose a synthesis pathway for the following conversion:
- Answer
-
1) (a) NaOH, H2O
(b) NaOH, H2O, then LiAlH4
(c) LiAlH42)

