19.13: Conjugate Nucleophilic Addition to α, β-unsaturated Aldehydes and Ketones
- Page ID
- 36393
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- explain how the carbonyl group which is present in α, β‑unsaturated aldehydes and ketones activates the carbon‑carbon double bond so that it is susceptible to attack by nucleophiles.
- write equations to illustrate the addition of amines, water and lithium diorganocopper reagents to α, β‑unsaturated aldehydes and ketones.
- identify the product formed from the reaction of a given primary or secondary amine with a given α, β‑unsaturated aldehyde or ketone.
- identify the aldehyde or ketone, the primary or secondary amine, or both, needed to prepare a given β‑amino aldehyde or ketone.
- identify the product formed from the reaction of an α, β‑unsaturated aldehyde or ketone with water.
- identify the product formed from the reaction of a given α, β‑unsaturated aldehyde or ketone with a given lithium diorganocopper reagent.
- identify the α, β‑unsaturated aldehyde or ketone, the lithium diorganocopper reagent, or both, needed to prepare a given product through a conjugate addition reaction.
At first this section may appear to contain a considerable amount of information, but you should realize that much of the material presented is really repetition. Essentially we see how three different nucleophilic reagents, primary and secondary amines, water and lithium diorganocoppers can add across a carbon‑carbon double bond when the latter is conjugated to the carbonyl group of an aldehyde or ketone. Note that the first reagent can also react directly with the carbonyl group of an aldehyde or ketone when there is no conjugated carbon‑carbon double bond present, but that the third reagent, lithium dialkylcopper, cannot do so.
You may be confused about the designation of the product from the conjugate addition to an α, β‑unsaturated aldehyde or ketone as a 1,4 adduct. You can more clearly understand this name if you recognize that the proton added in the second step of the reaction first adds to the oxygen of the enolate ion to produce an enol. The latter then tautomerizes to the more stable keto form.
One of the largest and most diverse classes of reactions involves nucleophilic additions to a carbonyl group. As discussed in Section 19.4, carbonyl carbons are electrophilic due to bond polarity created by resonance.
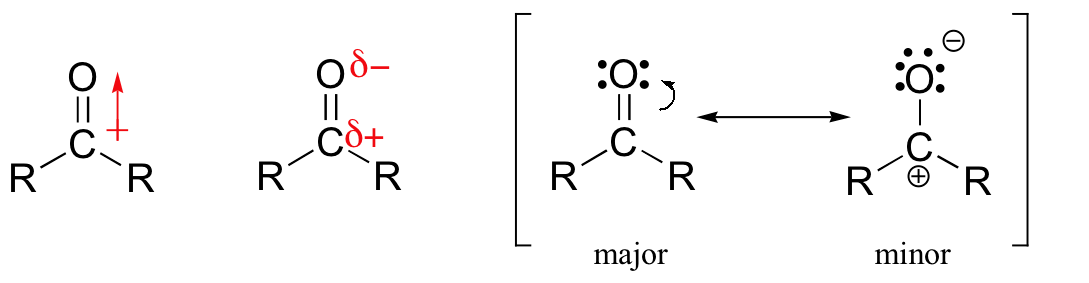
Previously in this chapter, we have discussed a nucleophilic addition to carbonyl carbons called a 1,2 addition. During 1,2 addition the nucleophile adds to the carbonyl carbon which is defined as the one position. Subsequently, hydrogen adds to the carbonyl oxygen which considered the two position. Overall an atom is added in both the 1 and 2 position justifying the reaction name, 1,2 addition.
Basic Reaction of 1,2 Addition
An important functional group is created when an alkene is placed in conjugation with a carbonyl. These conjugated carbonyl are called enones or α, β-unsaturated carbonyls. The term α commonly refers to the carbon adjacent to a carbonyl and β referrers to the next carbon in the chain.
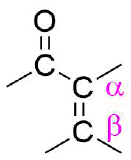
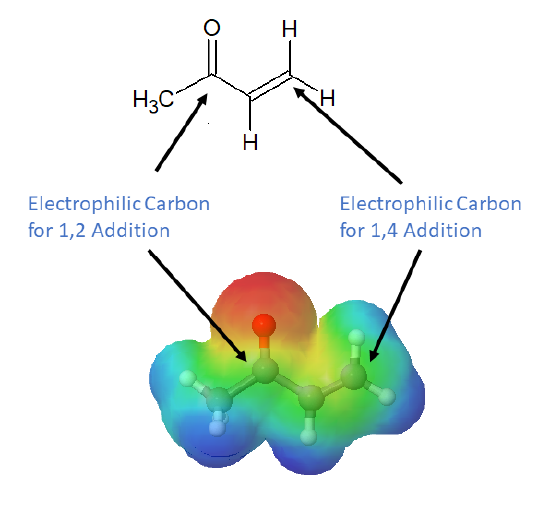
Conjugation transmits the electrophilic character of the carbonyl carbon to the β-carbon of the α, β-unsaturated carbonyl double bond. The resonance structure shown below shows that the electronegative oxygen atom in α, β-unsaturated carbonyls pulls electrons away from the β carbon making it more electrophilic than a typical alkene carbon.
From this resonance form, it should be clear that nucleophiles may attack either at the carbonyl carbon or at the β-alkene carbon. These two modes of reaction are referred to as 1,2-addition and 1,4-addition respectively. A 1,4-addition is also called a conjugate addition.
Basic Reaction of 1,4 Conjugate Addition
In 1,4 addition, a nucleophile is added to the carbon β to the carbonyl while a hydrogen is added to the carbon α to the carbonyl. Overall, the carbonyl is unaffected by the nucleophilic addition. It is important to note that this reaction only occurs because the alkene is conjugated with a carbonyl. The utility of 1,4 conjugate addition is shown by the wide variety of nucleophiles which can be added to a α, β unsaturated carbonyls.

General Mechanism for 1,4 Conjugate Addition
The mechanism starts with the nucleophile attacking the electrophilic β carbon forming a single bond. The two electrons from the alkene pi bond are pushed onto the electronegative carbonyl oxygen creating an enolate. In the next step the the enolate is protonated to form an enol. If the original nucleophile was neutral, this addition will cause it to become positively charge. A proton transfer will occur making the nucleophile neutral and turning the enolate into an enol. If the original nucleophile was negatively charged this protonation is accomplished by the subsequent addition of a proton source. The product of the second step of the mechanism shows why the reaction is called a 1,4 addition. The nucleophile bonds to the β alkene carbon which is considered the one position and the hydrogen adds to the carbonyl oxygen which is in the four position. Overall, addition occurs in the one and four position. In the final step of the mechanism, the enol undergoes a rearranges to form a carbonyl during a process called tautomerization. Tautomerization causes the hydrogen to move from the oxygen to the β-carbon. The tautomerization process will be discussed in greater detail in Section 22.3.
Step 1: Nucleophilic attack
Step 2: Protonation
Step 3: Tautomerization
Predicting the Products of a 1,4 Conjugate Addition.
In total, 1,4 addition occurs across the alkene bond of the α, β unsaturated carbonyl. The alkene pi bonds is broken to form two single bonds, one on the α-carbon and one on the β-carbon. During 1,4 addition, the α-carbon of the α, β unsaturated carbonyl forms a bond with a hydrogen while the β-carbon forms a bond to the nucleophile. Remember that neutral nucleophiles typically lose a hydrogen during 1,4 addition.
Nucleophiles Which add 1,4 to α, β Unsaturated Carbonyls
Hydroxide
Alcohols
Thiols
1o Amines
2o Amines
Cyanides
HBr
Examples
The synthesis of 3-(N-Methylamino)cyclohexanone
The Synthesis of 5-Hydroxy-3-heptenone
1,2 Vs. 1,4 Addition
Whether 1,2 or 1,4-addition occurs to a α, β unsaturated carbonyl depends on multiple variables but is mostly determined by the nature of the nucleophile. During the addition of a nucleophile there is a competition between the formation 1,2 and 1,4 addition products. If the nucleophile is a strong base, such as Grignard reagents or metal hydrides, both the 1,2 and 1,4 reactions are irreversible and therefor are under kinetic control. Since 1,2-additions to the carbonyl group are fast, we would expect to find a predominance of 1,2-products from these reactions. If the nucleophile is a weak base, such as, water, alcohols or amines, then the possible 1,2 addition is usually reversible. This means the competition between 1,2 and 1,4 addition is under thermodynamic control. In this most cases, the 1,4-addition product dominates because the stable carbonyl group is retained.
Nucleophiles Which add 1,2 to α, β-Unsaturated Carbonyls
Metal Hydrides (LiAlH4)
Grignard Reagents
Organolithium Reagents
Example
Gilman Reagents
Another important reaction exhibited by organometallic reagents is metal exchange. Organolithium reagents react with cuprous iodide to give a lithium diorganocopper reagent, which often is referred to as a Gilman reagent. Remember that organolithium reagents are formed by a reaction of lithium metal with an organohalide. Lithium diorganocopper reagents are considered a source of carbanion like nucleophiles similar to Grignard and Organolithium reagents. However, the reactivity of lithium diorganocuprate reagents is slightly different and this difference will be exploited in different situations. Diorganocuprate reagents are made from the reaction of two equivalents of an organolithium reagent and copper (I) iodide (CuI). The created lithium diorganocuprate reagent acts as a source of R:-
Lithium Diorganocopper (Gilman Reagent)
General Reaction
Example
Formation of Lithium Dimethylcopper
Reaction of 1,4 Conjugate Addition of Gilman Reagents to α, β Unsaturated Ketones
Lithium diorganocopper reagents, R2CuLi, undergo 1,4 conjugate addition when reacted with α, β Unsaturated ketones. Using lithium diorganocopper reagents allows for a wide range of organic groups to undergo this 1,4 conjugate addition including alkyl, aryl, and alkenyl groups. Because a C-C single bond is formed this reaction is an excellent method for adding to the carbon framework of a ketone.
Example
Mechanism for the 1,4 Conjugate Addition of Lithium Diorganocopper Reagents to α, β-Unsaturated Ketone
This mechanism is only slightly different than the general mechanism for 1,4 conjugate addition described above. The mechanism starts with the nucleophilc diorganocopper anion (R2Cu-) adding to the electrophilc β alkene carbon forming a Cu-C bond. The R group from the diorganocopper is then transferred to the β alkene carbon with elimination of a neutral organocopper species (RCu). Protonation of the enolate ion followed by tautomerization creates the final 1,4 addition product.
Synthesis of Ketones using 1,4 Conjugate Addition of Lithium Diorganocopper Reagents to α, β Unsaturated Ketones
When using retrosynthetic analysis to plan the synthesis of a ketone, remember this reaction allows for the formation of a C-C bond between third and fourth carbon away from a ketone. The carbonyl group in the target molecule will become a α, β unsaturated carbonyl in the probable reactant. To determine the structure of a possible reactant, start by cleaving the C-C between the third and fourth carbon away from the carbonyl to create two fragments. The take the fragment which retains the carbonyl and remove a hydrogen from the second carbon and connect the second and third carbon with a double bond. The creates the required α, β unsaturated carbonyl reactant. The other fragment will become the lithium diorganocopper reagent. Remember the lithium diorganocopper reagent contains two of the required R group. Note! If the fourth carbon away from the ketone in the target molecule is tertiary or quarternary there may be multiple bonds which could be retrosynthetically cleaved.
If you have ever encountered a metallic scent when counting coins or handling metals, especially copper, you were probably being fooled. The metallic smell could not have come from the metal because metals are non-volatile and do not evaporate. This means the odor molecules associated with metal scent must come from a different source. The volatile molecules that create the metallic smell are produced by a chemical reaction between skin oils and the metal itself. These volatile molecules, primarily aldehydes and ketones, create a sensory illusion that the metal is producing the smell, even though metals have no natural odor. The main molecule is the α, β Unsaturated Ketone, 1-Octen-3-one, which is described as smelling like mushrooms or metal. This is an illusion because the molecule doesn’t smell like metals; metals smells like this molecule. Because we have smelled 1-Octen-3-one coming from metals for so long, we mistake its smell with the metal itself. This kind of chemical reaction also explain why blood produces a metallic smell when it comes into contact with skin. Blood contains iron in hemoglobin, and the same chemical reaction produces odor molecules when blood touches skin.
Structure of 1-Octen-3-one
Problems
1) How would you make the following molecule using a 1,4 Conjugate Addition of a Gilman Reagen to a α, β Unsaturated Ketone?
2) Draw the bond-line structures for the products of the reactions below.
a)
b)
3) Specify the reagents needed to perform the following chemical transformation.
Solutions
1)
2)
a)
b)
3)

