19.4: Nucleophilic Addition Reactions of Aldehydes and Ketones
- Page ID
- 239308
Objectives
After completing this section, you should be able to
- give a general description of the nucleophilic addition reactions of aldehydes and ketones, identifying the two possible courses (or variations) that such reactions can take after the initial attack by the nucleophile.
- explain why, in general, aldehydes undergo nucleophilic addition reactions more readily than do ketones, and determine which of two given aldehydes or ketones will react most readily in such reactions.
Key Terms
Make certain that you can define, and use in context, the key term below.
- nucleophilic addition reaction
Study Notes
We have already discussed electrophilic addition reactions at some length; now you will meet nucleophilic addition reactions for the first time. Nucleophilic addition reactions involve the initial attack of a nucleophile on the slightly positive carbon centre of the carbonyl group.
Introduction
Before we consider in detail the reactivity of aldehydes and ketones, we need to look back and remind ourselves of what the bonding picture looks like in a carbonyl. Carbonyl carbons are sp2 hybridized, with the three sp2 orbitals forming three sigma bonds by overlapping with two s orbitals from the hydrogens and an sp2 hybrid obrital from oxygen. These three bonds adopt trigonal planar geometry seen in carbonyls. The carbonyl carbon's remaining unhybridized 2p orbital is perpendicular to this plane, and forms a ‘side-by-side’ pi bond by overlapping with a 2p orbital from the carbonyl oxygen.
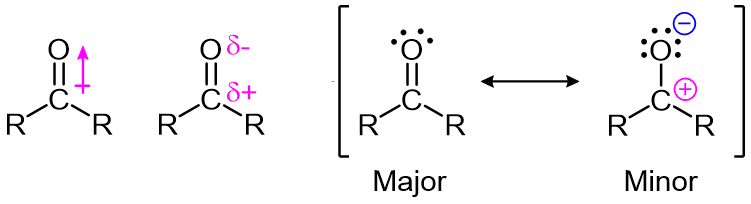
The carbon-oxygen double bond is polar: oxygen is more electronegative than carbon, so electron density is higher on the oxygen side of the bond and lower on the carbon side. Recall that bond polarity can be depicted with a dipole arrow, or by showing the oxygen as holding a partial negative charge and the carbonyl carbon a partial positive charge. A third way to illustrate the carbon-oxygen dipole is to consider the two main resonance contributors of a carbonyl group: the major form, which is what you typically see drawn in Lewis structures, and a minor but very important contributor in which both electrons in the pi bond are localized on the oxygen, giving it a full negative charge. The latter depiction shows the carbon with an empty 2p orbital and a full positive charge.
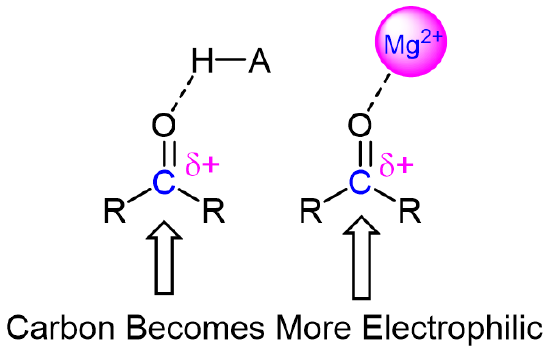
The result of carbonyl bond polarization, however it is depicted, is straightforward to predict. The carbon, because it is electron-poor, is an electrophile: it is a great target for attack by an electron-rich nucleophilic group. Because the oxygen end of the carbonyl double bond bears a partial negative charge, anything that can help to stabilize this charge by accepting some of the electron density will increase the bond’s polarity and make the carbon more electrophilic. Very often a general acid group serves this purpose, donating a proton to the carbonyl oxygen. The same effect can also be achieved if a Lewis acid, such as a magnesium ion (Mg2+), is located near the carbonyl oxygen
Nucleophilic Addition to a Carbonyl
Most important reactions involving carbonyl groups characteristically have a nucleophilic addition as part of their mechanism. The generic mechanism for the nucleophilic addition of a negatively charged nucleophile to a carbonyl is shown below.
In the first step of the nucleophilc addition mechanism, the nucleophile forms a bond with the the electrophilic C=O carbon atom. This causes the rehybridization of the carbonyl carbon from sp2 to sp3. Unlike SN2 nucleophilic substitution reactions, aldehyde or ketones has no leaving group so electrons in the pi bond are pushed up to the electronegative oxygen atom forming a tetrahedral alkoxide intermediate.
In the second step of the mechanism, the alkoxide is protonated by the addition of an acid to form an alcohol.
Stereochemistry of Nucleophilic Addition to a Carbonyl
Recall from Section 17.4 that when the two groups adjacent to a carbonyl are not the same, we can distinguish between the re and si 'faces' of the planar structure. The concept of a trigonal planar group having two distinct faces comes into play when we consider the stereochemical outcome of a nucleophilic addition reaction. Notice that in the course of a carbonyl addition reaction, the hybridization of the carbonyl carbon changes from sp2 to sp3, meaning that the bond geometry changes from trigonal planar to tetrahedral. If the two R groups are not equivalent, then a chiral center is created upon addition of the nucleophile. The configuration of the new chiral center depends upon which side of the carbonyl plane the nucleophile attacks from. Reactions of this type often result in a 50:50 racemic mixture of stereoisomers, but it is also possible that one stereoisomer may be more abundant, depending on the structure of the reactants and the conditions under which the reaction takes place.
Nucleophilic Addition of a Neutral Nucleophile
Neutral nucleophiles can also undergo nucleophilic addition to carbonyls. The mechanism is slightly different than with a negatively charged nucleophile. The presence of an additional hydrogen atom in the nucleophile allows for the the carbonyl oxygen to be completely removed as water and a C=Nu bond to be formed.
Relative Reactivity of Aldehydes and Ketones to Nucleophilic Addition
In general, aldehydes are more reactive than ketones because they have a greater polarization of the carbonyl bond. The primary carbocation formed in the in the polarizing resonance structure of an aldehyde (shown below) is less stable and therefore more reactive than the secondary carbocation formed in a similar resonance structure formed by a ketone. The stability difference in these resonance structures is due to the extra alkyl group present in the ketone. Alky groups stabilize carbocations through induction as discussed in Section 7.11.
This difference in the relative positive charge on the carbonyl carbon of an aldehyde and a ketone can be seen by comparing electrostatic potential maps of formaldehyde and acetone (Figure 1). The carbonyl carbon of formaldehyde is more positive (more blue) and therefore more reactive towards nucleophlic addition than the carbonyl carbon in acetone which is less positive (more green). As discussed above, electron-attracting groups can further increase its positive character of a carbonyl carbon and thereby facilitate nucleophilic addition.
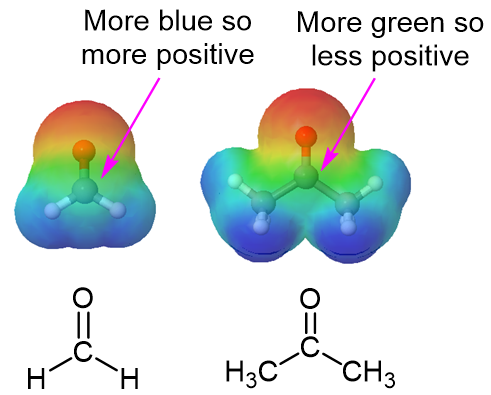
Figure 1: The Electrostatic Potential Map of Formaldehyde (Left) and Acetone (Right)
Another reason aldehydes tend to me more reactive to nucleophilic addition than ketones is sterically hinderance. Ketones have two alkyl groups attached to their carbonyl carbon while aldehydes only have one. This means nucleophiles have a less sterically hindered path when attacking the carbonyl carbon of an aldehyde. Also, the transition state of the rate determine step for formation of the the tetrahedral intermediate is less sterically crowded, lower in energy, and more kinetically favorable for an aldehyde than a ketone. The difference in sterics in the carbonyl carbon of an aldehde and a ketone is easily seen when comparing the space filling models of acetaldehyde and acetone (Figure 2).
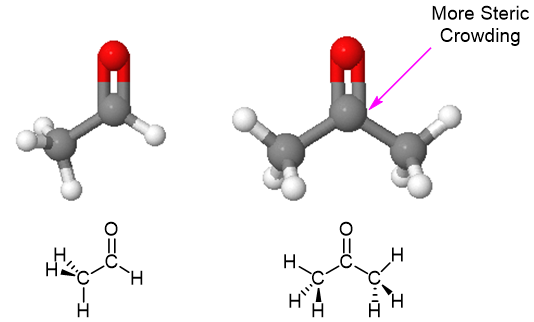
Figure 2: The Relative Steric Crowding of Acetaldehyde (Left) and Acetone (Right)
In fact, reactivity of a carbonyl toward nucleophilic addition decreases with increasing bulkiness of carbonyl substituents, as seen in the following series.
Problems
1) Benzaldehyde is less reactive to nucleophilic addition reactions than aliphatic aldehydes. Explain.
2) Compare the mechanisms of an SN2 reaction between 2-bromobutane and cyanide and the tetrahedral complex formation between 2-butanone and cyanide.
- Answers
-
1) Aromatic rings act as an electron-donating group due to resonance effect. Electron donating groups make the carbonyl group in benzaldehyde less electrophilic.
2) In the SN2 reaction the cyanide nucleophlic must undergo back side attack. This causes an inversion of stereochemistry and creates a product which is stereospecific. The carbonyl used in the nucleophilic addition is trigonal planar. This means the cyanide nucleophilie can attack from either plane. This tends to create a racemic mixture of enantiomers.
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)

