19.12: Biological Reductions
- Page ID
- 36392
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- determine whether or not a given aldehyde will undergo the Cannizzaro reaction.
- write an equation to illustrate the Cannizzaro reaction.
- identify the products formed when a given aldehyde undergoes a Cannizzaro reaction.
- write the detailed mechanism of the Cannizzaro reaction.
Make certain that you can define, and use in context, the key term below.
- Cannizzaro reaction
In 1853 Stanislao Cannizzaro discovered that the base‑induced disproportionation of an aldehyde resulted in a carboxylic acid (oxidation product) and an alcohol (reduction product).

Note that in the mechanism shown in the reading has an intermediate generated from methanal (formaldehyde) that effectively becomes a hydride (H−) donor, which reduces a second molecule of methanal to methanol.

Carbonyl reductions are a part of important biological pathways in living organisms. Recall in Section 17.4 that NADH can donate H− to reduce aldehydes and ketones. You may wish to review this section.
As seen in Section 18.11, nucleophilic addition reactions usually do not occur in many carboxylic acid derivatives due to the presence of a leaving group. Following nucleophilic addition, the tetrahedral alkoxide intermediate can reform the carbonyl by eliminating the leaving group as part of a reaction called nucleophilic acyl substitution. The nucleophilic acyl substitution reaction will be discussed in greater detail in Chapter 21.
This reaction does not occur in aldehydes or ketones because alkyl or hydrogen substituents make very poor leaving groups. After nucleophilic addition, the negative charge on the tetrahedral alkoxide intermediate remains on the oxygen and is subsequently protonated to form a hydroxyl group (OH). This represents the 1,2 nucleophilc addition reaction discussed in most of this chapter.
The Cannizzaro Reaction
An important exception to the idea of hydrogen making a poor leaving group is seen in the Cannizzaro reaction, discovered in 1853 by Stanislao Cannizzaro. A characteristic reaction of aldehydes without \(\alpha\) hydrogens is the self oxidation-reduction they undergo in the presence of strong base. If the aldehyde has \(\alpha\) hydrogens, other reactions usually occur more rapidly. The Cannizzaro reaction, combines many features of other reactions studied in this chapter. During this reaction, nucleophilic addition of a hydroxide (-OH) to an aldehyde gives a tetrahedral alkoxide intermediate. The alkoxide subsequently reforms the carbonyl, eliminating a hydride ion (-:H) as a leaving group, and creating an oxidized carboxylic acid product. The eliminated hydride ion reduces a second aldehyde molecule, in a similar fashion as hydride containing reagents LiAlH4 and NaBH4, to create a reduced alcohol product. For example, methanal creates methanol (reduced) and methanoic acid (oxidized) when heated with aqueous NaOH.
Example
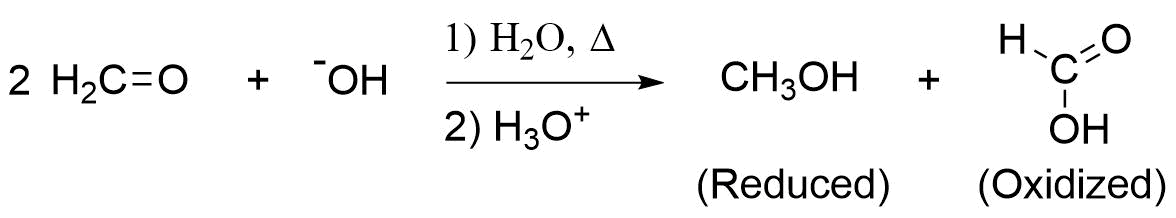
Methanal Undergoing a Cannizzaro Reaction to form Methanol and Methanoic Acid
Mechanism of the Cannizzaro Reaction
The first step of the mechanism is the reversible nucleophilic addition of a hydroxide ion to the carbonyl group of an aldehyde. This is analogous to the first mechanistic step in the formation of a hydrate under basic conditions. The hydroxide nucleophile attacks the electrophilic carbonyl carbon forming a C-O single bond. The two electrons from the carbonyl pi bond are pushed onto the electronegative oxygen to form a tetrahedral alkoxide intermediate. In the second step of the mechanism, the alkoxide intermediate reforms the carbonyl, eliminating a hydride ion as the leaving group, and forms a carboxylic acid functional group as an oxidized product. The hydride ion acts as a nucleophile and attacks the carbonyl carbon of a second aldehyde forming a C-H bond. The two electrons from the second pi bond are pushed onto the electronegative oxygen to form a different alkoxide intermediate. This alkoxide intermediate is quickly protonated to form an alcohol functional group as a reduced product.
1) Nucleophilic attack
2) Hydride transfer
Biological Reductions Using Hydride Transfer to a Carbonyls
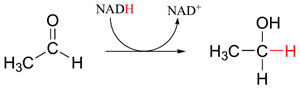
The Cannizzaro reaction is interesting because its mechanism is analogous to the hydride reductions of carbonyls which occur in the biological pathway of many living organisms. One of the most important biological reducing agents is NADH (the reduced form of nicotinamide adenine dinucleotide) which can act as a source of the hydride ion. NADH donates a hydride to carbonyls in an analogous fashion to the alkoxide ion intermediate of the Cannizzaro reaction. A set of lone pair electrons on a NADH nitrogen atom pushes the hydride off as a leaving group forming NAD+ (the oxidized form of nicotinamide adenine dinucleotide). The ejected hydride adds as a nucleophile to a wide variety of carbonyl containing biological molecules. Some examples of this addition are described below.
Nicotinamide Adenine Dinucleotide - a Hydride Transfer Coenzyme
Although we are talking about hydrides acting as nucleophiles and leaving groups in this section, you already know that discreet hydride ions are far too unstable to exist as actual intermediates in the organic reactions of living cells. Biochemical redox reactions involving hydride transfer require the participation of a hydride transfer coenzyme. In reactions involving carbonyl compounds, a molecule called nicotinamide adenine dinucleotide generally plays this role. The full structure of the oxidized form of this coenzyme, abbreviated NAD+, is shown below, with the active nicotinamide group colored blue.
Because the redox chemistry occurs specifically at the nicotinamide part of the molecule, typically the rest of the molecule is simply designated as an 'R' group. NAD+ and NADP+ both function in biochemical redox reactions as hydride acceptors: that is, as oxidizing agents. The other forms of the coenzyme, NADH and NADPH, serve as hydride donors: that is, as reducing agents.
The phosphate on the nucleotide pentose group of NADP+ and NADPH is located far from the nicotinamide ring, and does not participate directly in the hydride transfer function of the cofactor.
This reaction can be simplified in the following way:
Stereochemistry of Hydride Transfer Reactions
Ketone/aldehyde hydrogenase reactions are stereospecific in two distinct ways. In the hydrogenase direction, attack by the hydride can occur from either the re or the si face of an asymmetrical carbonyl, leading to the S or R alcohol depending on the priorities of R1 and R2 (in the example R1 has higher priority than R2).
The specificity is determined by which side of the ketone or aldehyde substrate the NAD(P)H cofactor is bound to in the active site. In addition, hydrogenases specifically catalyze the transfer of either the pro-R or the pro-S hydrogen at C4 of the nicotinamide ring. In the example below, the pro-R hydrogen of NADH is transferred to reduce the ketone.
Examples of Biological Hydride Transfer Reactions
- In 1997, an enzyme was discovered in a class of microbes known as ‘archaea’ that catalyzes the same reaction with the opposite substrate stereochemistry (J. Biochem 1997, 122, 572). The primary metabolic role of the enzyme is apparently to catalyze the reduction of dihydroxyacetone phosphate to (S)-glycerol phosphate, with transfer of the pro-R hydrogen atom from NADPH to the re face of dihydroxyacetone phosphate.
- In a reaction that is very important to people in the wine and beer-making industry, an NADH-dependent enzyme in yeast produces ethanol by reducing acetaldehyde. The acetaldehyde is derived from the thiamine diphosphate-dependent decarboxylation of pyruvate.
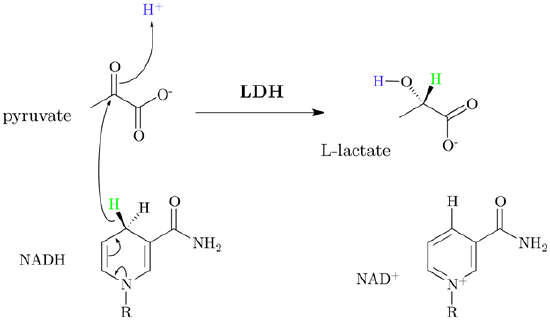
- Lactic acid fermentation occurs by converting pyruvate into lactate through hydride addition using the enzyme lactate dehydrogenase and producing NAD+ in the process. This process takes place in oxygen depleted muscle and some bacteria. It is responsible for the sour taste of sauerkraut and yogurt.
Problems
1) Please draw the expected products of the following reaction:
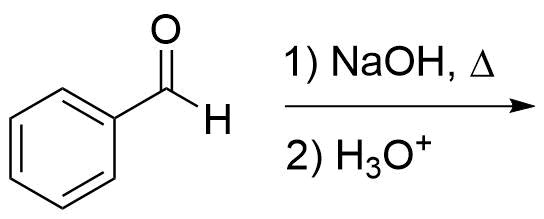
2) Please draw the mechanism for the following hydride transfer reaction:
Solutions
1)
2)

