19.11: Nucleophilic Addition of Phosphorus Ylides - The Wittig Reaction
- Page ID
- 36391
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- write an equation to illustrate the formation of an ylide (phosphorane).
- write an equation to illustrate the reaction that takes place between an ylide and an aldehyde or ketone.
- identify the alkene which results from the reaction of a given ylide with a given aldehyde or ketone.
- identify the aldehyde or ketone, the ylide, or both, needed to prepare a given alkene by a Wittig reaction.
Make certain that you can define, and use in context, the key terms below.
- betaine
- Wittig reaction
- ylide (phosphorane)
The name triphenylphosphine is derived as follows: the compound PH3 is called phosphine; replacing the three hydrogen atoms with phenyl groups therefore gives us triphenylphosphine.
Note the following series of IUPAC‑accepted trivial names:
- NH3—ammonia
- PH3—phosphine
- AsH3—arsine
Phosphorus Ylides
An ylide, is defined as a compound with positive and negative charges on adjacent atoms and an overall neutral charge. There are multiple types of ylides but for the Wittig reaction an organophosphorus ylide, also called a Wittig Reagent, will be used.
Although many Wittig reagents are commercially available, it is often necessary to create them synthetically. Wittig reagents can be synthesized from an alkylphosphonium salt created using an SN2 reaction between an alkyl halide and a nucleophilic trialkyl phosphine. Because trialkyl phosphines typically make good nucleophiles, the SN2 reactions usually occur with high yields. The subsequent alkylphosphonium salt has an increased acidity (pKa ~22) and can be deprotonated using strong bases, such as n-butyllithium, sodium amide, sodium hydride, and alkoxides to create the neutral Wittig reagent. The increased acidity of the alkylphosphonium salt is due to the stabilizing inductive and resonance effects provided by the phosphorus adjacent to the carbanion conjugate base.
The ability of phosphorus to hold more than eight valence electrons allows for a resonance structure to be drawn forming a double bonded structure call a phosphorane. Although Wittig reagents are commonly drawn in the phosphorane form, the ylide form is often used because it shows the nucleophilic carbanion.
Mechanism of Ylide Formation
The lone pair electrons on the phosphine attack the electrophilic carbon of an alkyl halide as part of a SN2 reaction. This forms a P-C bond while ejecting the halogen leaving group to create an alkylphosphonium salt. A strong base, such as n-butyllithium, deprotonates the acidic hydrogen of the alkylphosphonium salt to create an ylide Wittig reagent.
Step 1: SN2 Reaction
Step 2: Deprotonation
Example
A common Wittig reagent is methylenetriphenylphosphorane (Ph3P=CH2) which is synthesized by reacting Triphenyl phosphine with methylbromide followed by deprotonation with n-butyllithium.
Ylides in Synthesis
Because an SN2 reaction is used in the synthesis of ylides, methyl and primary halides perform the best. Secondary halides can also be used but the yields are generally lower. This should be considered when planning out a synthetic pathway which involves a synthesized Wittig reagent.
The ylides are typically strong bases and are protonated by water, alcohols, and other acidic hydrogens. Contact with water causes phosphorous ylides to decompose in to hydrocarbons and phosphine oxides, as shown below.
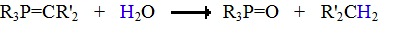
The Wittig Reaction
Alkylidenephosphorane ylides (Wittig Reagents) react with aldehydes or ketones through nucleophilic addition, to give substituted alkenes in a reaction called the Wittig reaction. This reaction was developed in 1954 by George Wittig who was awarded the Nobel Prize for this work in 1979. Because of its reliability and wide applicability, the Wittig reaction has become a standard tool used in organic synthesis for the preparation of alkenes.
In the Wittig reaction, an ylide (typically triphenylphosphorus ylide), adds to an aldehyde or ketone to yield a four-membered heterocyclic intermediate called an oxaphosphetane. The oxaphosphetane intermediate spontaneously decomposes to produce an alkene plus a phosphine oxide. Cleavage of the oxaphosphetane to alkene and phosphine oxide products is exothermic and irreversible.
Predicting the Product of a Wittig Reaction
Overall the carbonyl oxygen of an aldehyde or ketone is replaced by the nucleophilic, carbanion present in the ylide while still maintaining the double bond. First, convert the oxygen in the starting material into a carbon while maintaining the double bond. This carbon represents the nucleophilic carbon in the ylide. Then look at the ylide reactant and observe the two groups attached to the nucleophilic carbon. Then transfer these to the bare carbon in the double bond. There may be some possibility of creating E/Z isomers and this will be discussed later.
Examples of the Wittig Reaction
Mechanism of the Wittig Reaction
The initial mechanistic step to form the oxaphosphetane intermediate appears to take place by different pathways which is dependent on the structure of the reactants and the exact experimental conditions. One pathway involves a [2+2] cycloaddition between the ylide and carbonyl to directly form the oxaphosphetane. Cyloadditions, such as this, will be discussed in more detail in section 30.5.
In another postulated mechanism, the ylide undergoes nucleophilic addition to the carbonyl group, forming the initial carbon-carbon bond and creating a dipolar charge-separated intermediate species called a betaine. The betaine subsequently undergoes ring closure to form the four-membered oxaphosphetane structure. In the final step of the mechanism, the oxaphosphetane undergoes intramolecular elimination to give an alkene and a triphenylphosphine oxide.
Step 1: Nucleophillic attack on the carbonyl double bond.
Step 2: Formation of the 4-membered oxaphosphetane ring.
Step 3: Intramolecular elimination
Benefits of the Wittig Reaction
A principal advantage of alkene synthesis by the Wittig reaction is that the location of the double bond is absolutely fixed, in contrast to the mixtures often produced by alcohol dehydration or other elimination reactions.
Limitations of the Wittig Reaction
One limitation of the Wittig reaction is possibility of E and Z isomers of the alkene forming. With simple ylides, the product formed is usually primarily the Z-isomer, although a lesser amount of the E-isomer is often also formed. If the E-isomer is the desired product, variations of the Wittig reaction, such as the Schlosser modification which uses a stabilized ylide, may be used. It is common to avoid the possibility of forming E/Z isomer by either using symmetrical ketones or symmetrical ylides.
As mentioned above, the Wittig reagent itself is usually derived from an SN2 reaction with an alkyl halide. Ylide formation with most secondary halides is inefficient. For this reason, Wittig reagents are rarely used to prepare tetrasubstituted alkenes. However, the Wittig reaction is robust and can tolerate the presence of many other functional groups including alkenes, aromatic rings, ethers, alcohols, and ester groups. Bis-ylides (containing two P=C bonds) have also been made and used successfully.
Synthesis of Alkenes Using the Wittig Reaction
When using retrosynthetic analysis to determine the preferred route to synthesize an alkene, it is important to remember that Wittig reagents are commonly prepared by using an SN2 reaction. To find the possible reactants of a Wittig reaction, cleave the C=C bond in the target molecule to create two pieces. Place an oxygen on one piece to create a carbonyl and place a (Ph3)P on the other piece to create the Wittig reagent. There are two possible combinations of this separation. The one whose Wittig reagent has the fewest number of alkyl groups will be easier to prepare by the required SN2 reaction and will represent the preferred route.
The Wittig reaction is commonly used in the preparations of pharmaceuticals and other commercial chemicals. The dietary source of vitamin A (β-Carotene) can be synthesized by a Wittig reaction using two equivalents of the ylide, β-ionylideneacetaldehyde.
Exercises
1) Draw the product of the following reactions.
2) Draw the structure of the oxaphosphetane which is made during the mechanism of the reaction given that produces product C.
3) Draw the structure of the betaine which is made during the mechanism of the reaction given that produces product D.
4) Indicate the starting material required to produce the product in each reaction.
5)Give a detailed mechanism and the final product of this reaction
6) It has been shown that reacting and epoxide with triphenylphosphine forms an alkene. Please propose a mechanism for this reaction. Review the section on epoxide reactions if you need help.
Answers
1)
2)
3)
4)
5)
Nucleophillic attack on the carbonyl
Formation of a 4 membered ring
Formation of the alkene
6)
Nucleophillic attack on the epoxide
Formation of a 4 membered ring
Formation of the alkene

