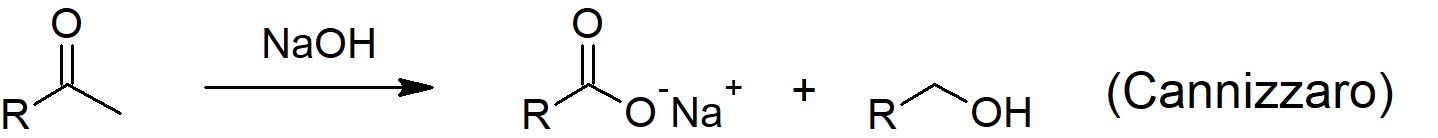19.S: Aldehydes and Ketones (Summary)
- Page ID
- 207035
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Concepts & Vocabulary
19.0 Chapter Objectives and Preview of Carbonyl Chemistry
- Aldehydes are carbonyl compounds with an R group and a hydrogen attached to the carbonyl carbon.
- Ketones are carbonyl compounds with two R groups attached to the carbonyl carbon.
19.1 Naming Aldehydes and Ketones
- Aldehydes are named following IUPAC rules with the standard ending -al.
- Ketones are named following IUPAC rules with the standard ending -one.
- Aldehydes have priority over ketones when both appear in the same molecule.
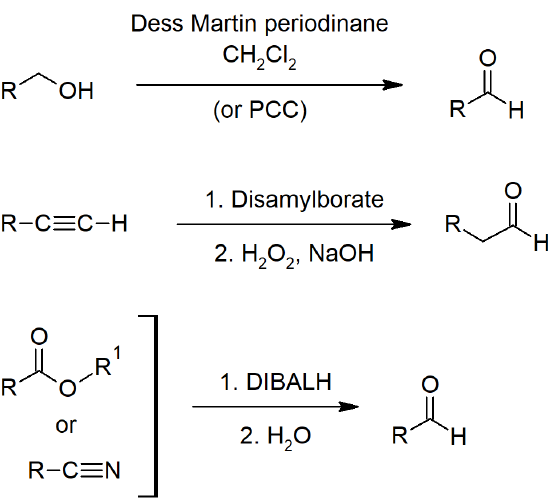
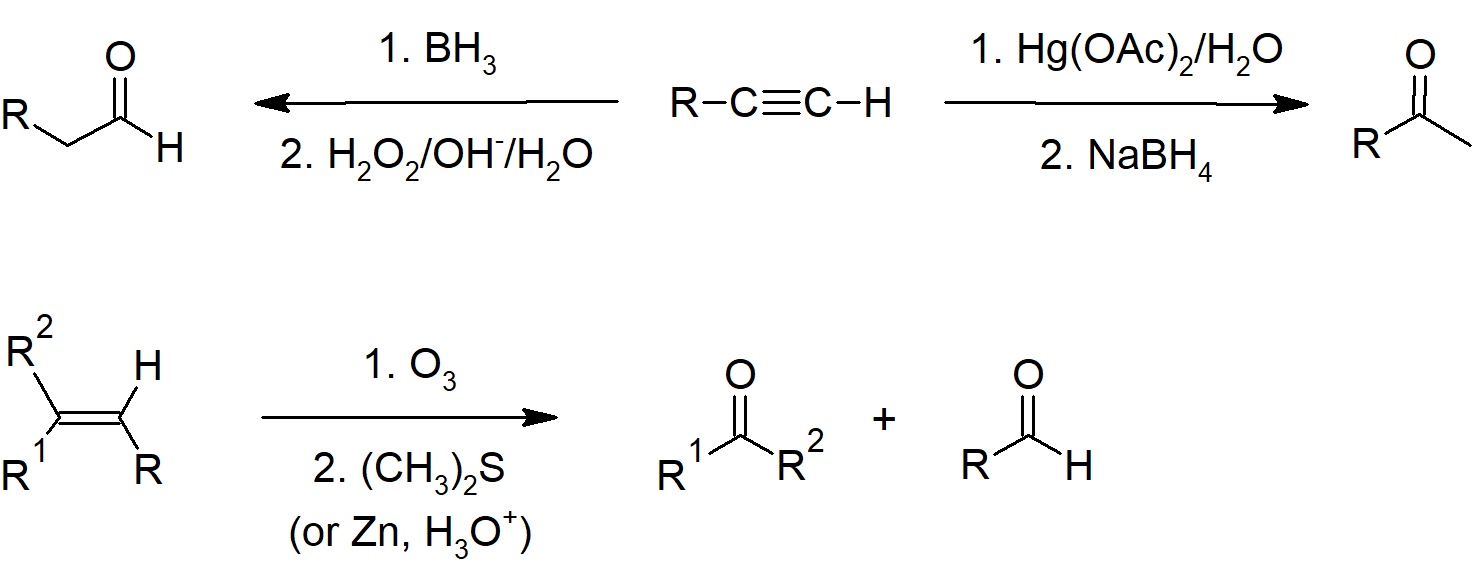
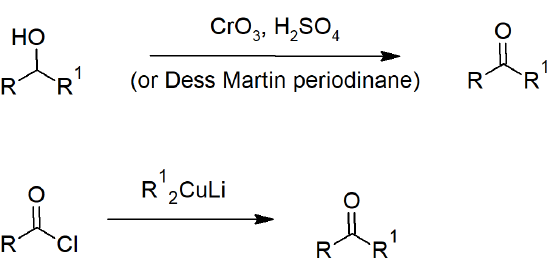
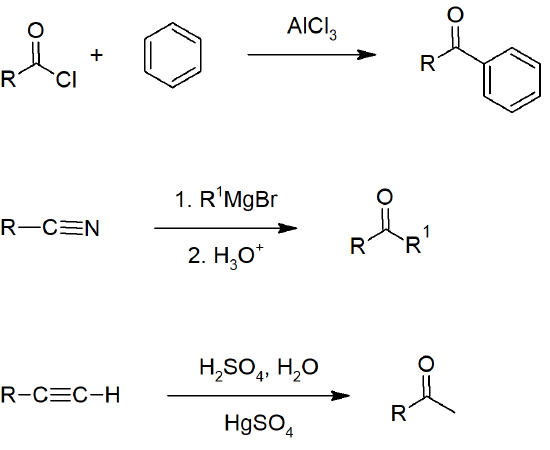
19.2 Preparing Aldehydes and Ketones
- Primary alcohols can be oxidized to aldehydes with PCC (pyridinium chlorochromate) or DMP (Dess-Martin Periodinane).
- Terminal alkynes can be hydrated to form aldehydes through hydroboration-oxidation reactions.
- Esters can be reduced to aldehydes with DIBAL-H.
- Acid chlorides can be reduced to aldehydes with hydrides.
- Nitriles can be reduced to aldehydes with DIBAL-H.
- Secondary alcohols can be oxidized to ketones with chromic acid/Jones reagent.
- Alkynes can be hydrated to form ketones.
- Aryl ketones can be formed by Friedel-Crafts acylation of aromatic rings.
- Nitriles can be reacted with Grignard reagents to form ketones.
- Alkenes can be cleaved with ozone to form aldehydes or ketones.
- Acid halides can be converted to ketones by reacting with Gilman reagents.
19.3 Oxidation of Aldehydes and Ketones
- Aldehydes can be oxidized more easily than ketones due to the aldehyde hydrogen.
- Jones reagent is a mixture of CrO3 and aqueous acid.
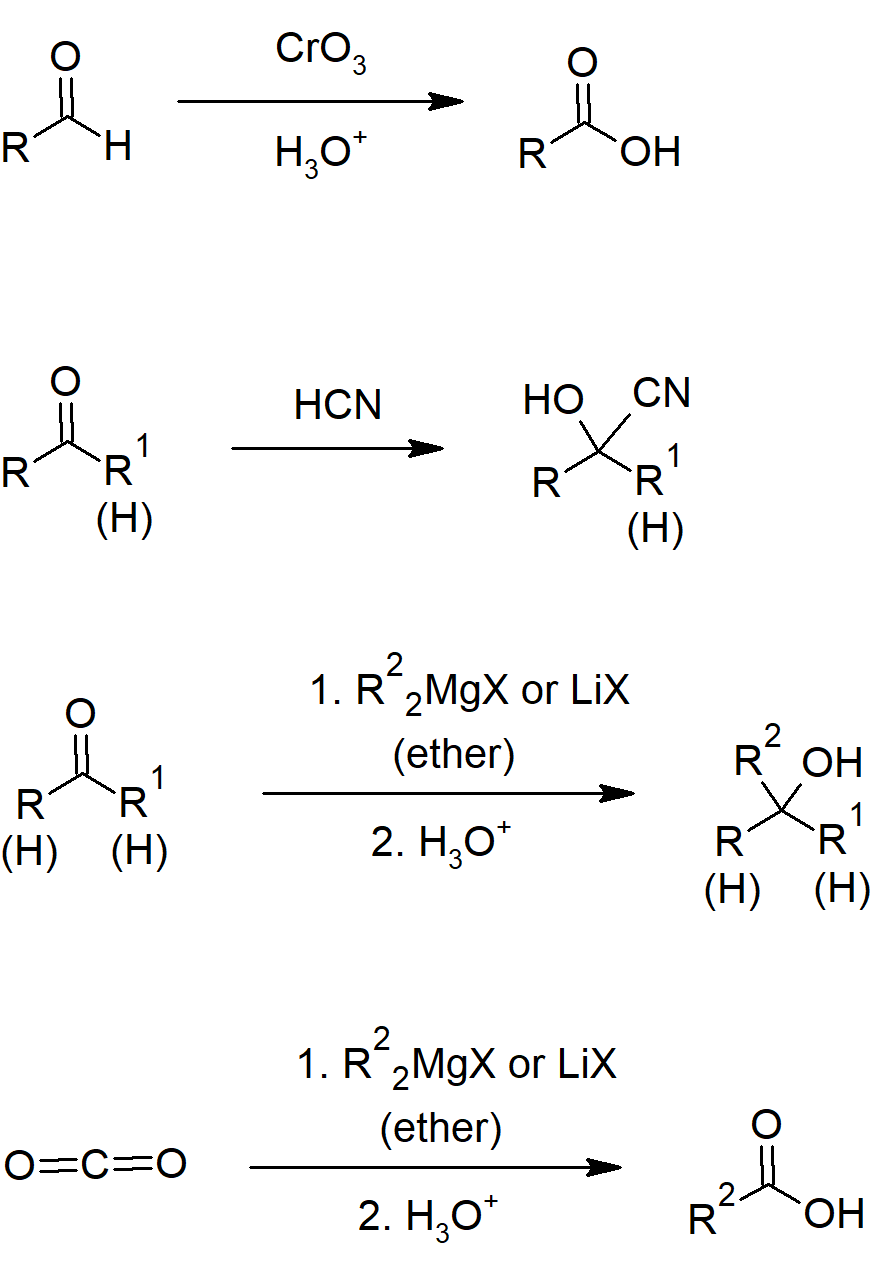
- Aldehydes can be oxidized to carboxylic acids with chromium trioxide/Jones reagent.
- Ketones can undergo oxidative cleavage with very strong oxidizing agents such as potassium permanganate.
- Ketones can be oxidized to esters by peroxycarboxylic acids in a reaction known as Baeyer-Villiger oxidation.
19.4 Nucleophilic Addition Reactions of Aldehydes and Ketones
- Carbonyl bonds are polarized with a partial positive charge on the carbon making it an electrophile and a target for attack by nucleophiles.
- Neither aldehydes nor ketones have a good leaving group causing both to undergo nucleophilic addition reactions.
- Aldehydes are more reactive than ketones to nucleophilic addition reactions.
19.5 Nucleophilic Addition Reactions of Water: Hydration
- Gem-diols are molecules that have two hydroxide groups attached to the same carbon.
- Aldehydes and ketones can react with water under either acidic or basic conditions to form gem-diols.
19.6 Nucleophilic Addition Reactions of HCN: Cyanohydrin Formation
- Cyanohydrins are molecules with a cyanide and a hydroxide attached to the same carbon.
- Cyanohydrins can be formed from aldehydes or ketones by reacting with cyanide ion and a weak acid.
19.7 Nucleophilic Addition Reactions of Hydride and Grignard Reagents: Alcohol Formation
- Metal hydrides can reduce aldehydes and ketones to alcohols.
- Organometallic reagents include carbon bonds to metals which react similarly to carbanions.
- Grignard reagents (R-Mg-X) and organolithium compounds add to aldehydes and ketones to form alcohols.
19.8 Nucleophilic Addition of Amines: Imine and Enamine
- Imines are characterized by a carbon-nitrogen double bond.
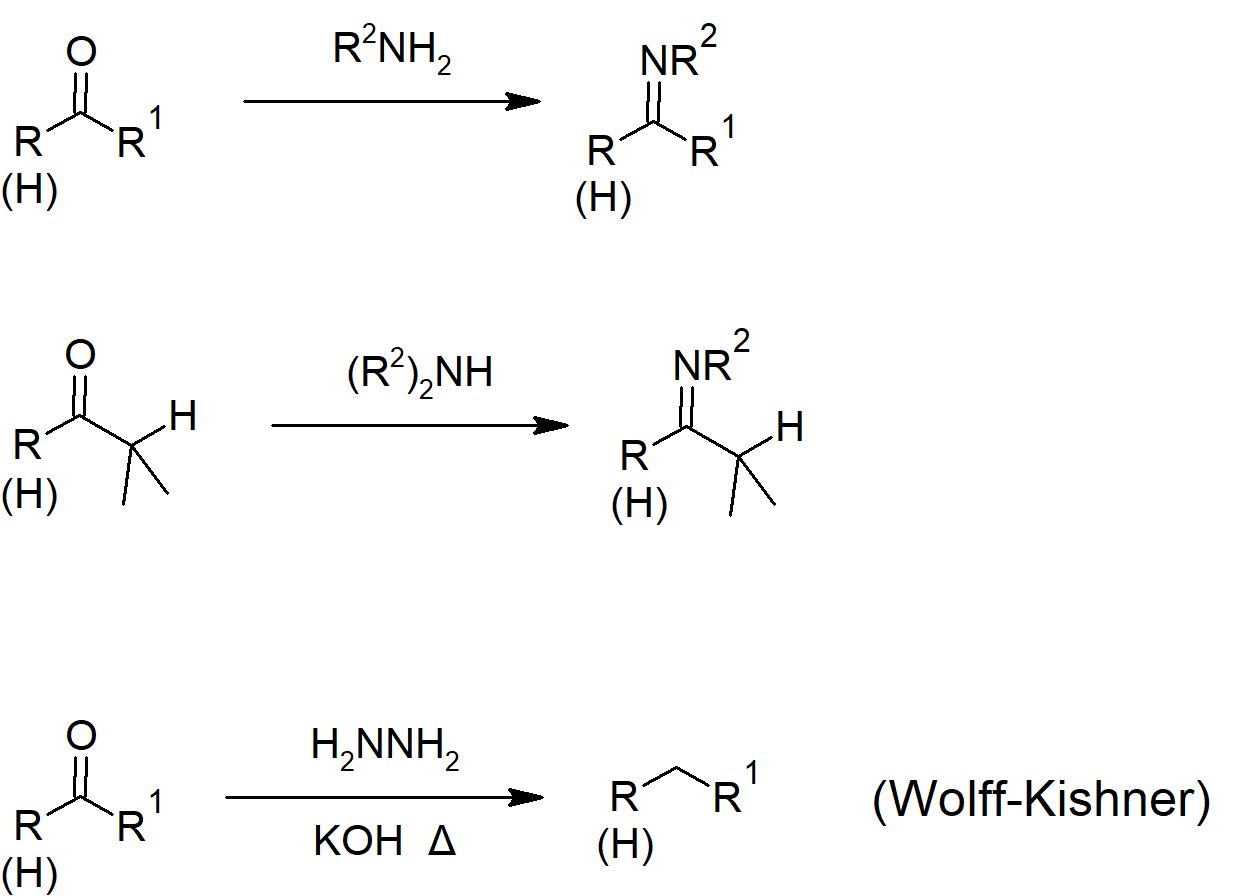
- Reaction of aldehydes and ketones with primary amines can form imines.
- Reaction of aldehydes and ketones with secondary amines can form enamines.
19.9 Nucleophilic Addition of Hydrazine - The Wolff-Kishner Reaction
- Hydrazine will react with aldehydes and ketones to form hydrazones (similar to imine formation). Heating of the hydrazone with base converts it to an alkane.
- The combination of hydrazone formation and reduction (of aldehydes and ketones) is called the Wolff-Kishner reaction.
19.10 Nucleophilic Addition of Alcohols: Acetal Formation
- Hemiacetals have one hydroxide and one ether attached to a carbon.
- Acetals have two ethers attached to a carbon.
- Reaction of an aldehyde or ketone with an alcohol under acidic conditions forms a hemi-acetal. Continuation of the same reaction results in an acetal.
- Acetals can reform the aldehyde or ketone by reacting with acid.
- Acetals are useful as protecting groups due to the reversible nature of acetal formation.
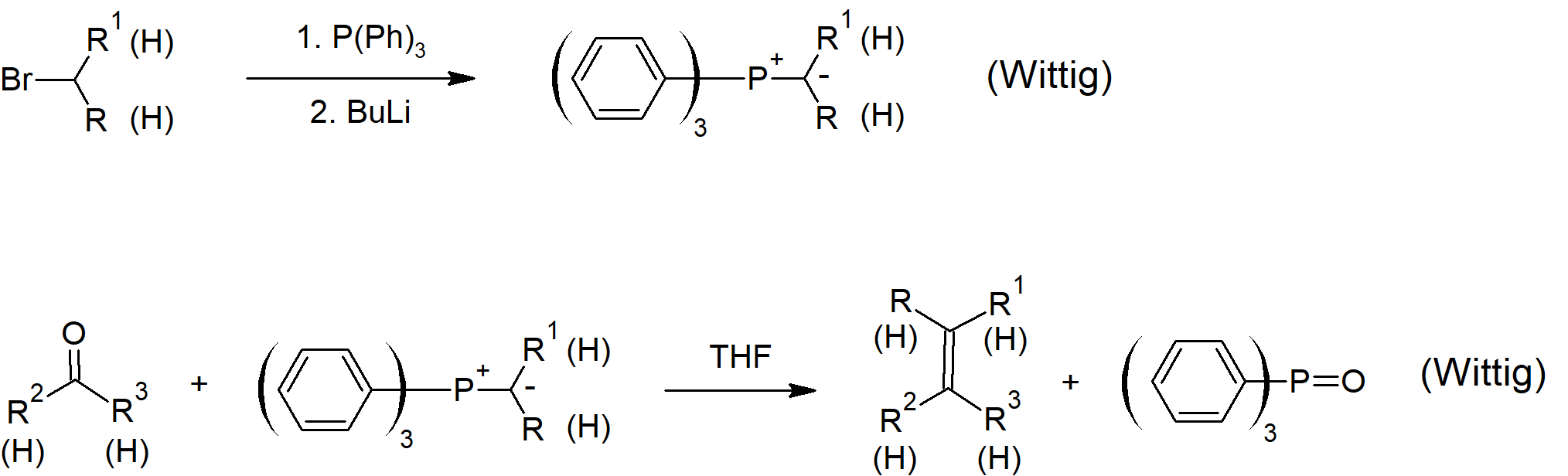
19.11 Nucleophilic Addition of Phosphorun Ylides: The Wittig Reaction
- A molecule with positive and negative charges on adjacent atoms is called an ylide.
- Wittig reagents are organophosphorus ylides which can be drawn as a double bonded structure called a phosphorane.
- The Wittig reaction is used to convert an aldehyde or ketone into an alkene by reacting with an organophosphrous ylide.
- Oxaphosphetane intermediates containing a 4 membered ring including one oxygen and one phosphorus atom occur during the Wittig reaction.
- The Cannizzaro reaction allows an aldehyde to react with another like molecule in strong base to form one oxidized molecule (carboxylic acid) and one reduced molecule (alcohol).
- NADH, one of the most important biological reducing agents, uses a similar mechanism to the Cannizzaro reaction while reducing ketones in biological systems to alcohols while being converted to NAD+.
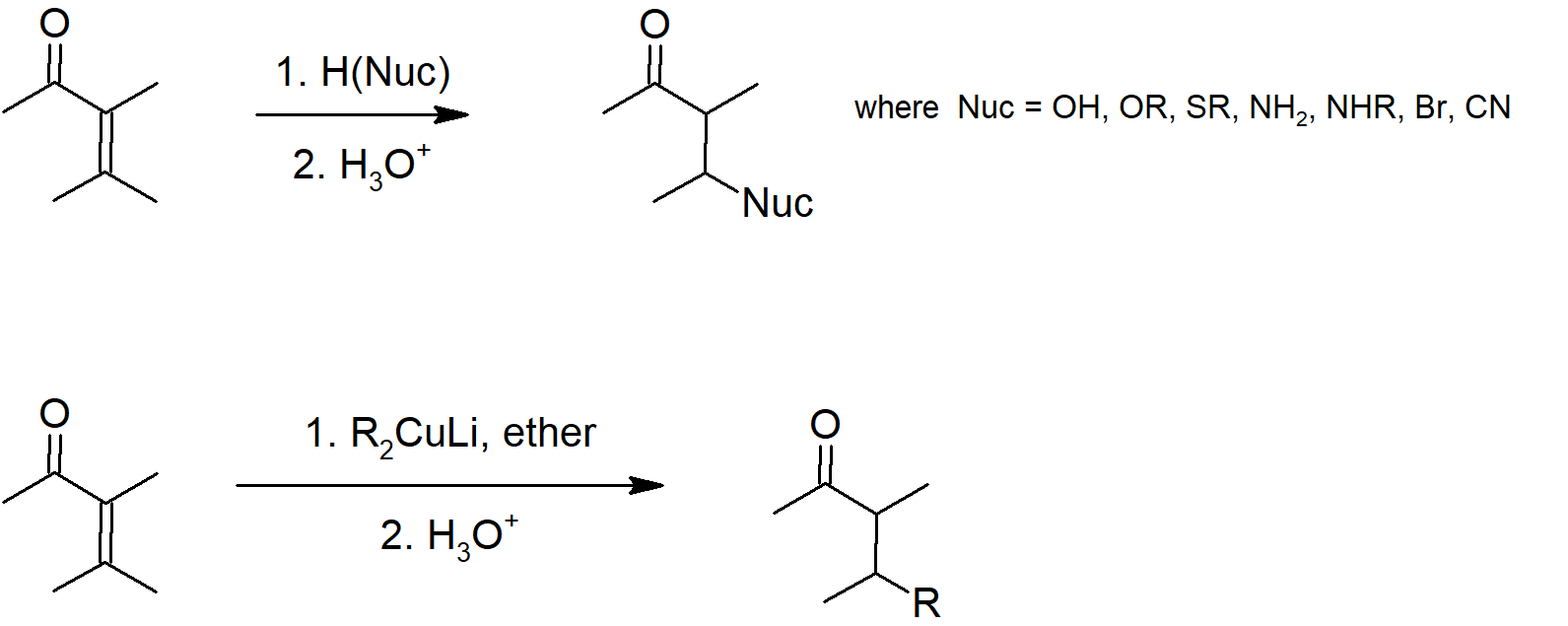
19.13 Conjugate Nucleophilic Addition of alpha, beta-Unsaturated Aldehydes and Ketones
- α, β‑unsaturated carbonyls have an alkene attached to the carbonyl carbon.
- Due to resonance delocalization, the β‑carbon of an α, β‑unsaturated carbonyl is electrophilic and will react with nucleophiles.
- α, β‑unsaturated carbonyls can undergo 1,2 or 1,4 addition.
- 1,4 addition is also called conjugate addition.
- Lithium diorganocopper reagents are called Gilman reagents.
- Gilman reagents typically react with α, β‑unsaturated carbonyls via conjugate addition.
19.14 Spectroscopy of Aldehydes and Ketones
- IR of aldehydes and ketones is defined strongly by the carbonyl stretching vibration.
- 1H NMR of aldehydes show the aldehyde proton between 10 and 11 ppm as well as the protons on carbon adjacent to the carbonyl at about 2.5 ppm.
- 1H NMR of ketones show the protons on carbon adjacent to the carbonyl at about 2.5 ppm.
- 13C NMR of aldehydes and ketones show the carbonyl carbon at about 200 ppm.
- Mass spectra of aldehydes and ketones typically yield moderately intense molecular ions, M+. They also can undergo some rearrangements that yield common fragmentation patterns.
Skills to Master
- Skill 19.1 Explain the relative reactivity of aldehydes vs. ketones to nucleophiles, based on carbonyl bond polarity.
- Skill 19.2 Name aldehydes and ketones using IUPAC rules.
- Skill 19.3 Draw the structure of an aldehyde or ketone from the IUPAC name.
- Skill 19.4 Interpret common names of aldehydes and ketones.
- Skill 19.5 Describe methods for preparing aldehydes and ketones.
- Skill 19.6 Draw mechanisms for oxidations of aldehydes and ketones.
- Skill 19.7 Explain general reactivity of aldehydes and ketones through the nucleophilic addition mechanism.
- Skill 19.8 Draw mechanisms for nucleophilic addition reactions to aldehydes and ketones including
- Hydration
- Cyanohydrin formation
- Hydride Reduction
- Organometallic reactions
- Addition of amines
- Wolff-Kishner reaction
- Acetal formation
- Wittig reaction
- Cannizzaro reaction
- Conjugate addition
- Skill 19.9 Use IR, NMR and MS to identify aldehydes and ketones.