18.2: Preparing Ethers
- Page ID
- 36366
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- write an equation to illustrate the industrial preparation of simple symmetrical ethers.
- write an equation to illustrate the Williamson synthesis of ethers.
- identify the ether obtained from the reaction of a given alkyl halide with a given alkoxide ion.
- identify the reagents needed to prepare a given ether through a Williamson synthesis.
- identify the limitations of the Williamson synthesis, and make the appropriate choices when deciding how best to synthesize a given ether.
- write an equation to describe the formation of an alkoxide from an alcohol.
- identify silver(I) oxide as a reagent which can be used in a Williamson synthesis.
- write an equation to show how an ether can be prepared by the alkoxymercuration-demercuration of an alkene.
- identify the product formed from the alkoxymercuration-demercuration of a given alkene.
- identify the alkene, the reagents, or both, needed to prepare a given ether by the alkoxymercuration-demercuration process.
- write the detailed mechanism of the reaction between an alkene, an alcohol and mercury(II) trifluoroacetate.
Make certain that you can define, and use in context, the key terms below.
- alkoxymercuration
- oxymercuration
- Williamson ether synthesis
We studied oxymercuration as a method of converting an alkene to an alcohol in Section 8.5. “Alkoxymercuration” is a very similar process, except that we are now converting an alkene into an ether. The two processes are compared below.
| Description | oxymercuration | alkoxymercuration |
|---|---|---|
| we react an | alkene | alkene |
| with | water | an alcohol |
| in the presence of | Hg(O2CCH3)2 | Hg(O2CCF3)2 |
| followed by treatment with | NaBH4 | NaBH4 |
| to produce an | alcohol | ether |
Review the mechanism of the oxymercuration reaction in Section 8.5, paying particular attention to the regiochemistry and the stereochemistry of the reaction. The mechanism is identical to alkoxymercuration.
Ether Formation Though Dehydration
Acid-catalyzed dehydration of small 1º-alcohols constitutes a specialized industrial method of preparing symmetrical ethers. This reaction cannot be employed to prepare unsymmetrical ethers because a mixture of products is likely to be obtained. Also, 2o and 3o alcohols cannot be used for this reaction because they dehydrate to form alkenes by an E1 mechanism (Section 17-6).
\[\ce{2 CH_3CH_2-OH + H_2SO_4 ->[130\;^oC] CH_3CH_2\bond{-}O\bond{-}CH_2CH_3 + H_2O} \tag{18.2.1} \]
Mechanism
In the first step of the reaction mechanism, one alcohol is protonated to become a good leaving group. In the second step, a second alcohol displaces water from the protonated alcohol during an SN2 reaction yielding a protonated ether. In the final step, this intermediate is deprotonated to yield the symmetrical ether.
Williamson Ether Synthesis
One important procedure, known as the Williamson Ether Synthesis, proceeds by an SN2 reaction of an alkoxide nucleophile with a primary alkyl halide or tosylate. As previously discussed in Section 17-2, alkoxides are commonly created by deprotonating an alcohol with a strong base, such as sodium hydride (NaH). Simple alcohols can be used a solvent during a Williamson ether synthesis and with their alkoxide created through the addition of sodium metal (Na(s)).
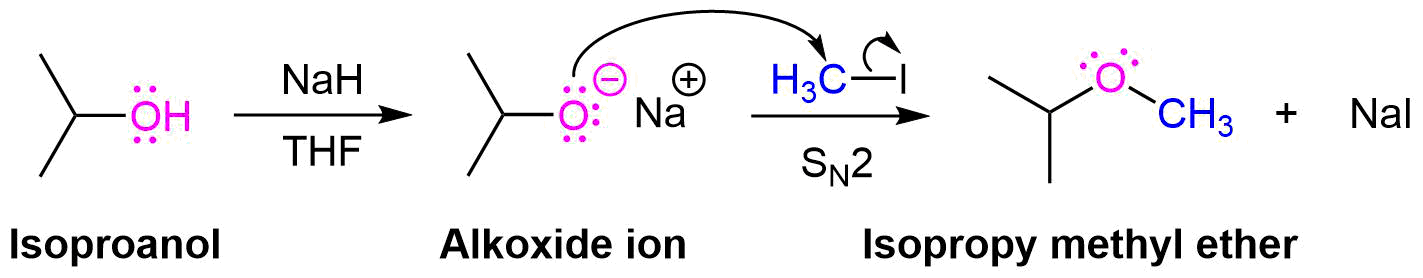
Planning a Williamson Ether Synthesis
The Williamson ether synthesis has the same limitations as other SN2 reactions, as discussed in Section 11-3. Since alkoxide anions are strong bases, utilizing 2o or 3o halogen leaving groups could possibly produce an E2 elimination product. When considering the synthesis of an unsymmetrical ether, there are two different combinations of reactants possible and each should be carefully considered. In general, the pathway which utilizes the least sterically hindered halogen will be preferred.
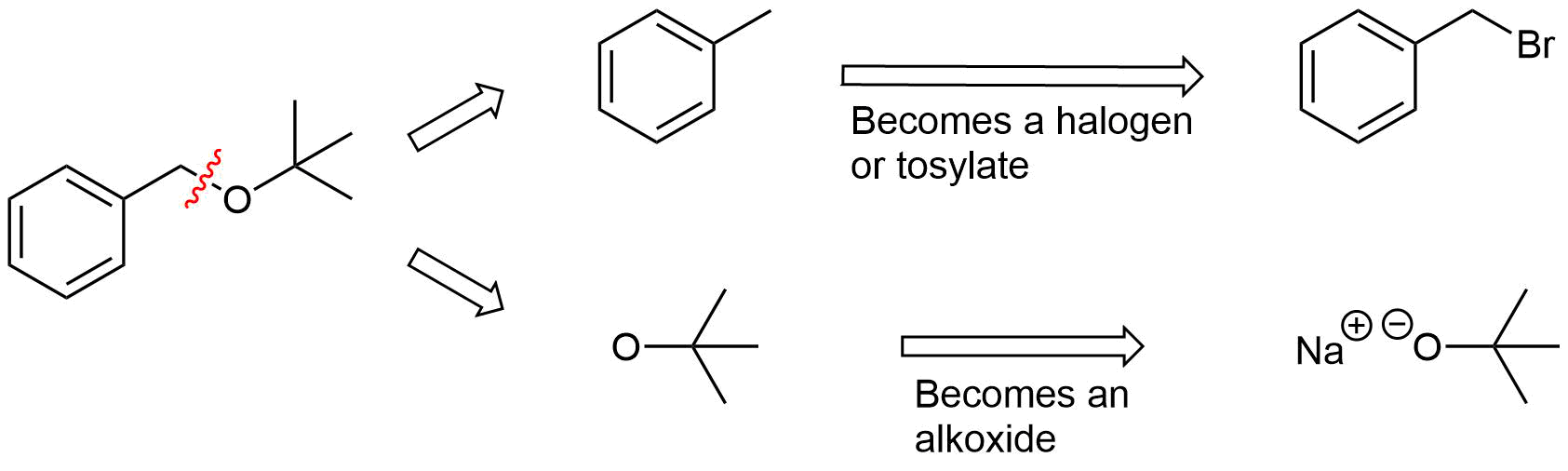
The key bond cleavage in the target molecule involves a C-O bond. Because unsymmetrical ethers have two unique C-O bonds, each can be broken to provide a unique set of reactants. After cleavage, the fragment with the oxygen will become an alkoxide. The other fragment will become a halogen or tosylate.
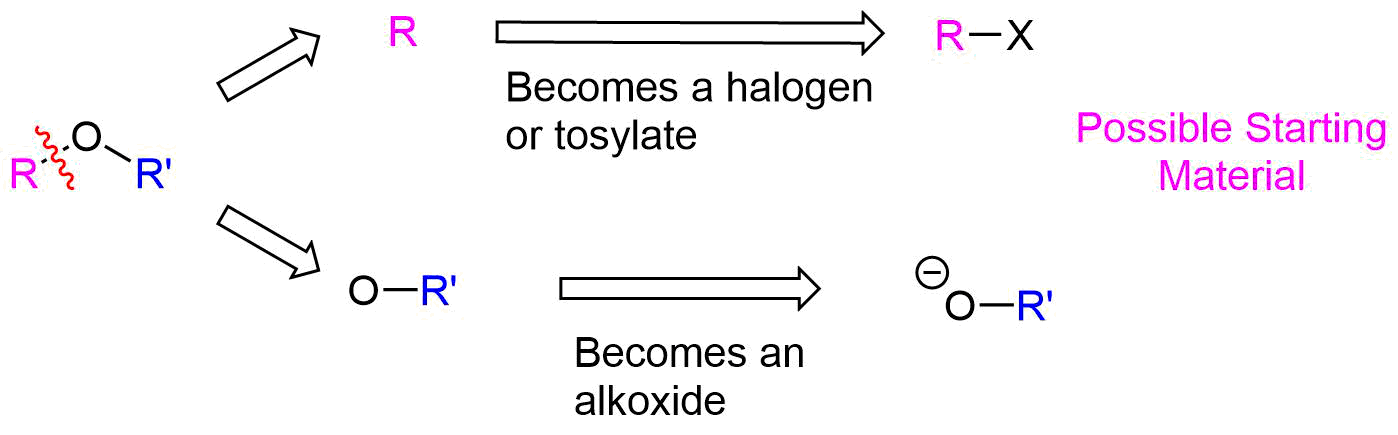
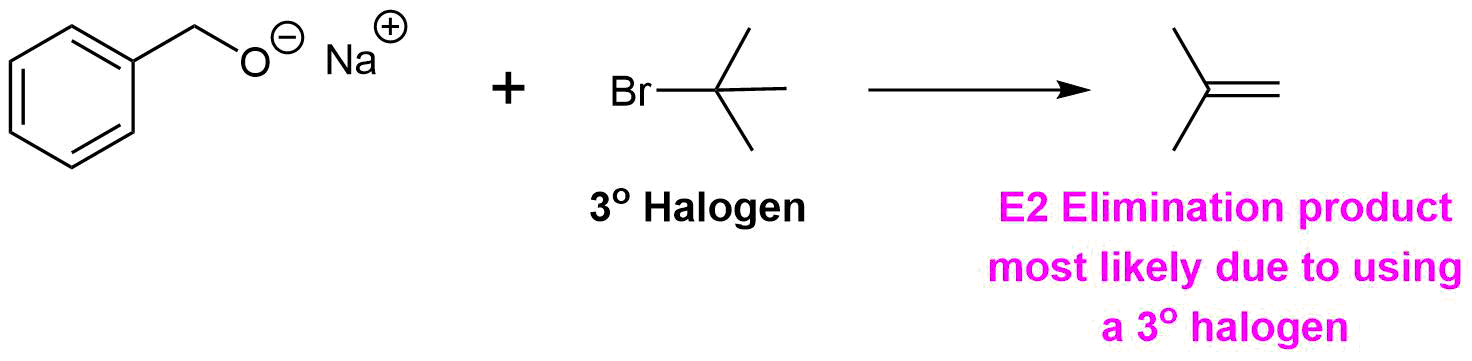
How would you prepare the following molecule using a Williamson Ether Synthesis?
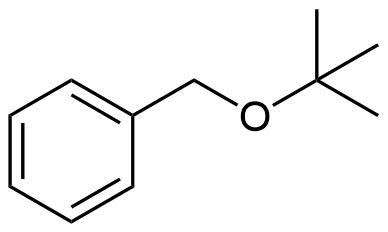
- Answer
-
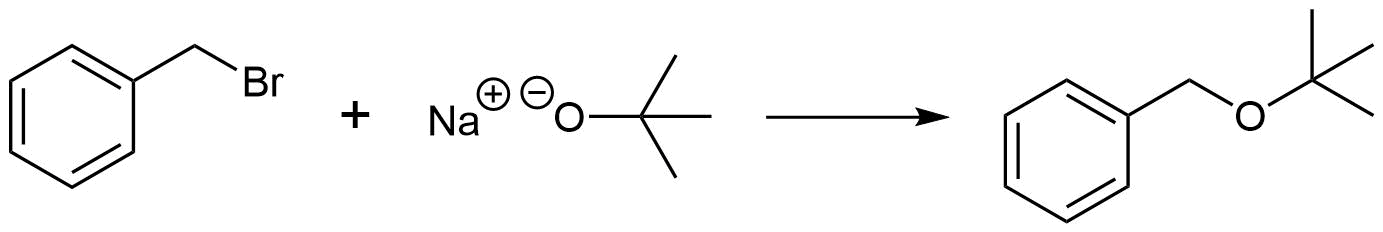
Analysis: The ether is asymmetrical so each of the C-O bonds can be broken to create a different set of possible reactants. After cleavage of the C-O bond, pathway 1 shows a 3o halogen as the starting material. This reaction will most likely not be effective due to alkoxides reacting with 3o halogens to preferable form an alkenes by E2 elimination. Pathway 2 shows a 1o halogen as a starting material which is favorable for SN2 reactions.
Pathway 1
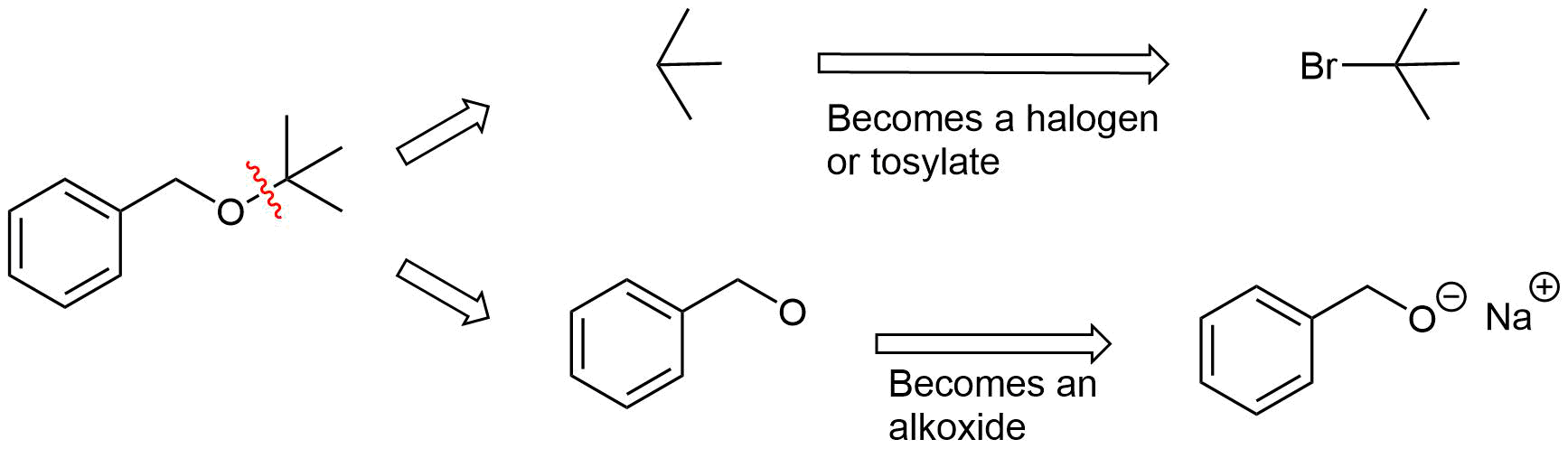
Solution 1
Pathway 2
Solution 2
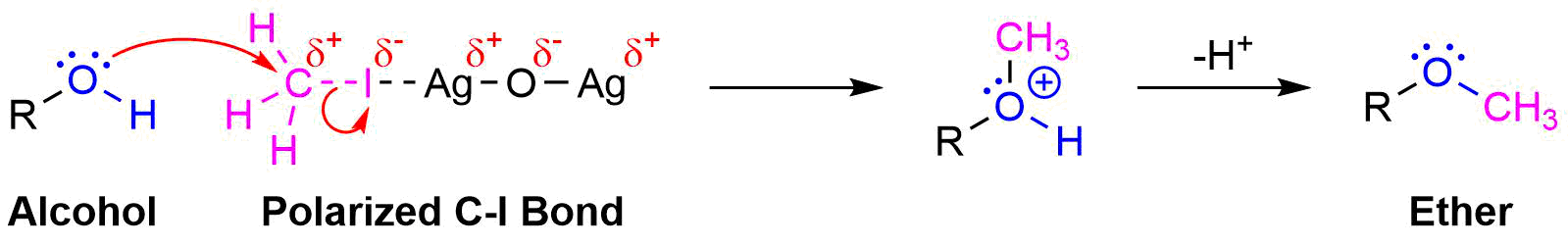
Ether Synthesis Using Silver Oxide
A variation of the Williamson ether synthesis uses silver oxide (Ag2O) in the place of the strong base. The conditions of this variation are milder than the typical Willamson synthesis because a strong base and the formation of an alkoxide intermediate are not necessary. This reaction is particually useful when converting the -OH groups on a sugar into ethers.
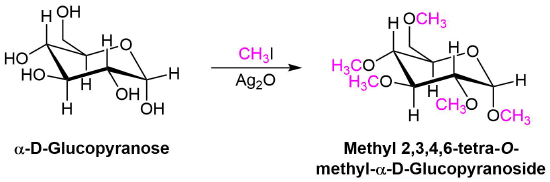
Mechanism
During this reaction a partial positively charged silver in Ag2O gives draws electron density from the iodine in CH3I. This correspondingly removes electron density from the adjacent carbon increasing its partial positive charge which increases its electrophlicity. This allows the alcohol to act as a nucleophile in the subsequent SN2 reaction.
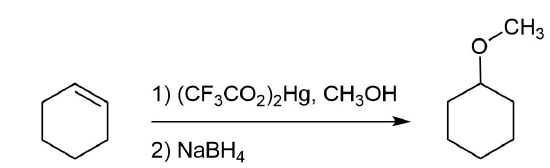
Ether Synthesis Using Alkoxymercuration
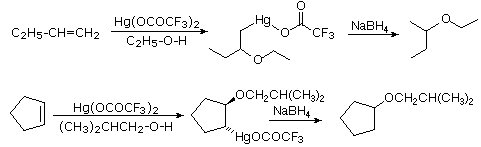
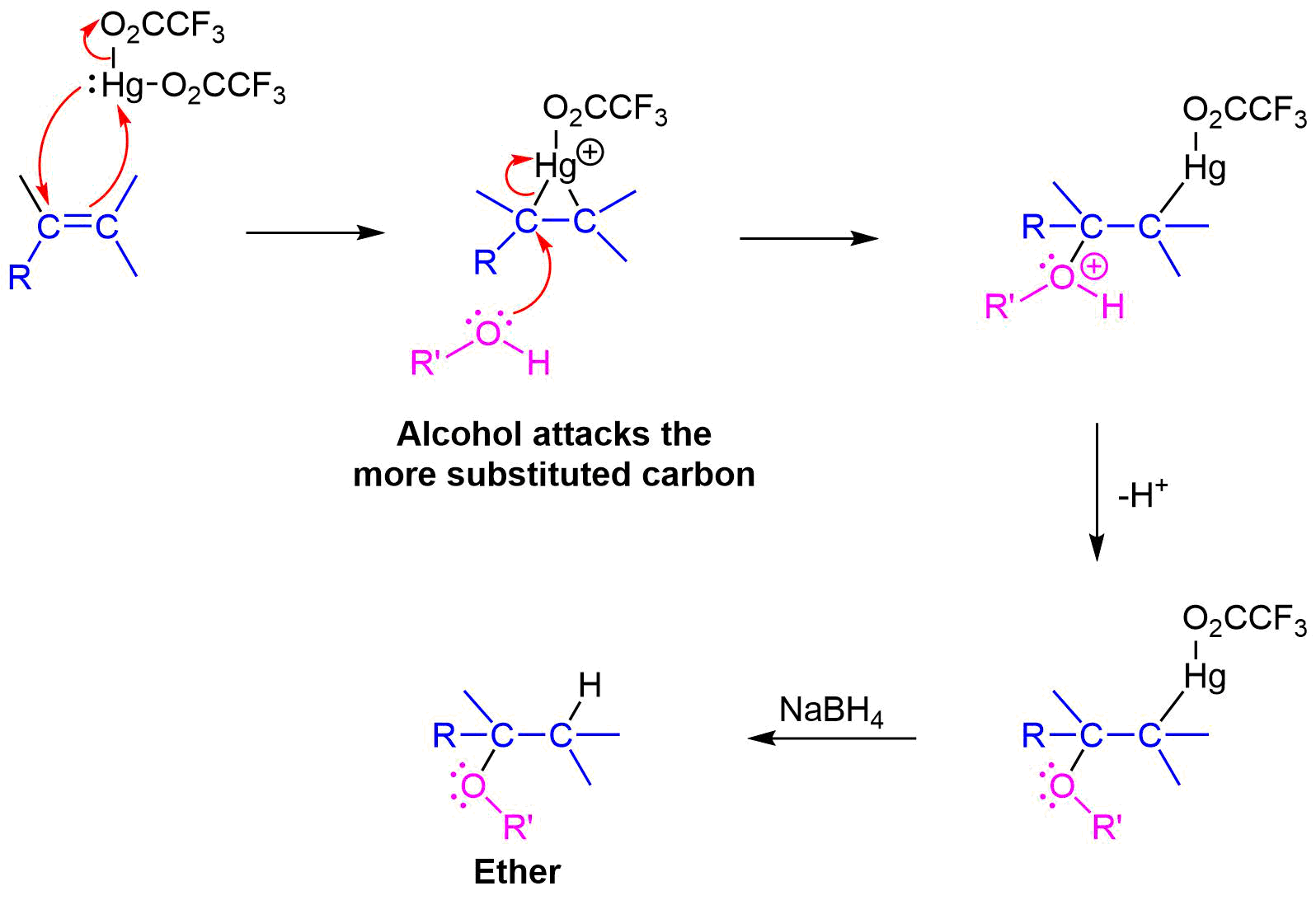
Alkoxymercuration, is patterned after the oxymercuration reaction discussed in Section 8-4. Reaction of an alkene with an alcohol in the presence of a trifluoroacetate mercury (II) salt [(CF3CO2)2Hg] prodcues an alkoxymercuration product. Demercuration using sodium borohydride (NaBH4) yields an ether product. Overall, this reaction allows for the Markovnikov addition of an alcohol to an alkene to create an ether. Note that the alcohol reactant is used as the solvent, and a trifluoroacetate mercury (II) salt is used in preference to the mercuric acetate (trifluoroacetate anion is a poorer nucleophile than acetate). Most 1o, 2o, 3o alcohols can be successfully used for this reaction.
Mechanism
The mechanism of alkoxymercuration is similar to that of oxymercuration, with electrophillic addition of the mercuric species to the alkene. The alcohol nucleophile attacks the more substituted carbon of the three-membered ring via a SN2 reaction. Finally, sodium borohydride (NaBH4) provides a reductive demercuration to form the ether product.
Planning the Synthesis of an Ether using Alkoxymercuration
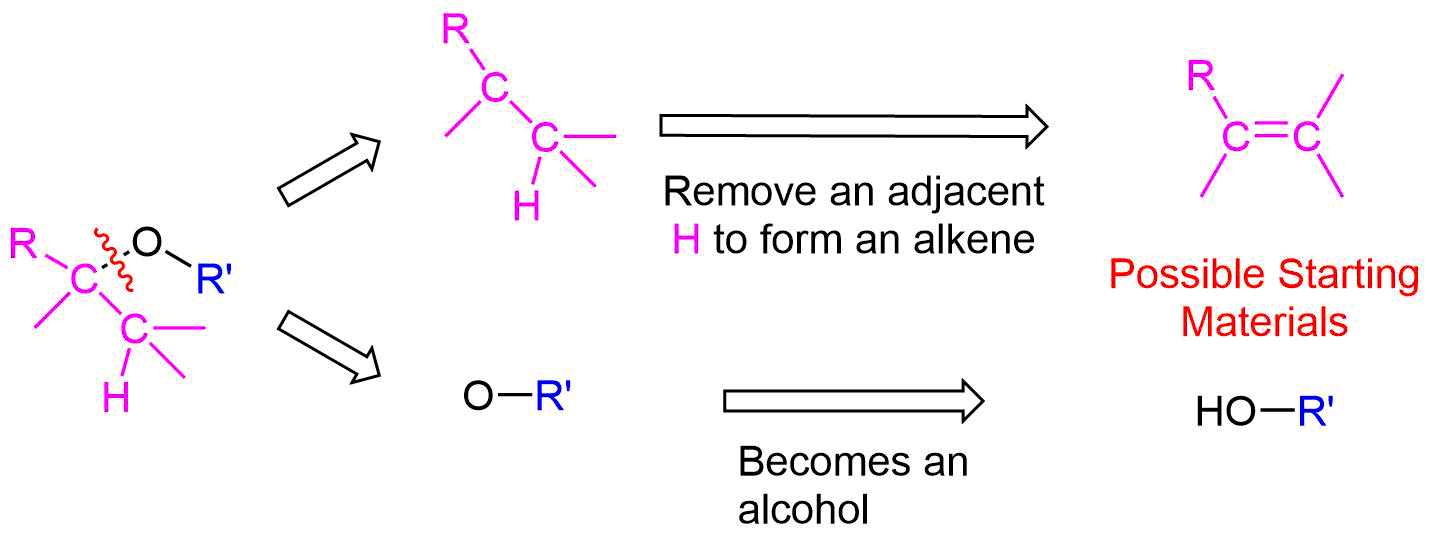
The key bond cleavage in the target molecule involves a C-O bond. Because unsymmetrical ethers have two unique C-O bonds, each can be broken to provide a unique set of reactants. After cleavage, the fragment with the oxygen will become an alcohol. The alkyl fragment will lose a hydrogen from a adjacent carbon to form an alkene. The main point to consider when choosing a possible synthesis pathways is the ability of the alkyl fragment to form an alkene.
How would you prepare the following molecule using a alkoxymercuration?
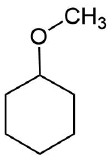
- Answer
-
Analysis: The ether is symmetrical so each C-O bond of the ether can be cleaved to produce a set of starting materials for consideration. Pathway one shows a set of starting material which should work well for this reaction. The alcohol, methanol, can easily be used as a solvent. Although the alkene does not have a defined more and less substituted side, its symmetry will prevent a mixture of product from forming. The fragmentation for pathway 2 shows starting material which are not viable for this reaction. The alkyl fragment only has one carbon which cannot be used to form an alkene starting material. This means pathway 2 is not a viable method for the synthesis of the target molecule.
Pathway 1
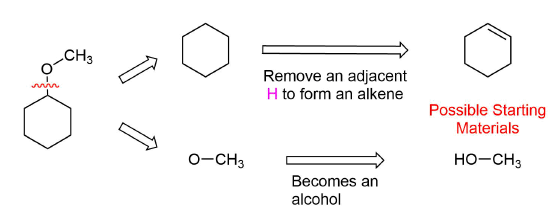
Solution 1
Pathway 2
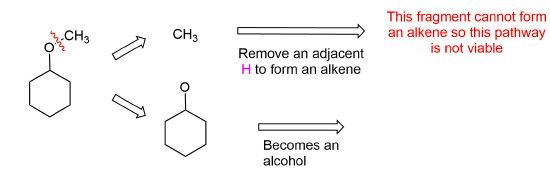
Exercises
When preparing ethers using the Williamson ether synthesis, what factors are important when considering the nucleophile and the electrophile?
- Answer
-
The nucleophile ideally should be very basic, yet not sterically hindered. This will minimize any elimination reactions from occurring. The electrophile should have the characteristics of a good SN2 electrophile, preferably primary to minimize any elimination reactions from occurring.
How would you synthesize the following ethers? Keep in mind there are multiple ways. The Williamson ether synthesis, alkoxymercuration of alkenes, and also the acid catalyzed substitution.
(a) (b)
(c)
(d)
(e)
- Answer
-
The Williamson ether syntheses require added catalytic base. Also, most of the halides can be interchanged, say for example for a -Br or a -Cl. Although, typically -I is the best leaving group.
(a)
(b)
(c)
(d)
Note, there is only one ether (also called a silyl ether, and often used as an alcohol protecting group.) The other group is an ester.
(e)
Draw the electron arrow pushing mechanism for the formation of diethyl ether in the previous problem.
- Answer
-
.
t-butoxycyclohexane can be prepared two different ways from an alkene and an alcohol, draw both possible reactions.
- Answer
-
While both are possible, the top route is likely easier because both starting materials are a liquid.
Epoxides are often formed intramolecularly. Take for example this large ring, in a publication from 2016 [J. Org. Chem., 2016, 81 (20), pp 10029–10034]. If subjected to base, what epoxide would be formed? (Include stereochemistry)
- Answer
-
What reagents would you use to perform the following transformations?
(a)
(b)
(c)
- Answer
-
(a)
(b)
Note the cis addition
(c)
An oxidation to an alcohol through hydroboration, and subsequent substitution with 2-bromopropane could also work, but this route provides the least likelihood of an elimination reaction occurring.
Predict the product of the following.
- Answer
-
The result is the production of dioxane, a common solvent.

