18.1: Names and Properties of Ethers
- Page ID
- 36365
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- write two acceptable names for a simple dialkyl ether, given its Kekulé, shorthand or condensed structure.
- name a complicated ether by the IUPAC system, given its Kekulé, shorthand or condensed structure.
- draw the Kekulé, condensed or shorthand structure of an ether, given an acceptable name.
- explain why the boiling point of an ether is generally higher than that of an alkane of similar molecular mass.
Structure of Ethers
Ethers are a class of organic compounds that contain an sp3 hybridized oxygen between two alkyl groups and have the formula R-O-R'. These compounds are used in dyes, perfumes, oils, waxes and other industrial uses. Aliphatic ethers have no aryl groups directly attached to the ether oxygen.
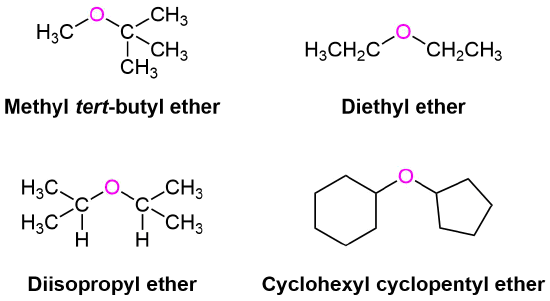
Examples of Aliphatic Ethers
Aromatic ethers have at least one aryl ring directly attached to the ether oxygen. In aryl ethers, the lone pair electrons on oxygen are conjugated with the aromatic ring which significantly changes the properties of the ether.
Example of Aromatic Ethers
The sp3 hybridization of oxygen gives ethers roughly the same geometry as alcohols and water. The R-O-R' bond angle is close to what is expected in a tetrahedral geometry. The bond angle of dimethyl ether is 112o which is larger than the H-O-H bond angle in water (104.5o) due to the steric repulsion of the methyl groups.
The presence of an electronegative oxygen atom gives ethers a small dipole moment with the electron density primarily on oxygen (red and orange in the electrostatic potential map).
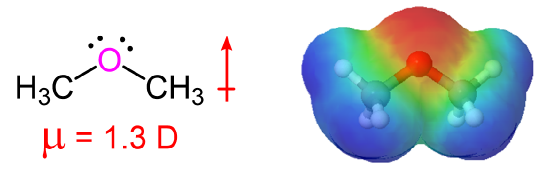
Comparisons of Physical Properties of Alcohols and Ethers
Ethers, unlike alcohols, have no hydrogen atom on the oxygen atom (that is, no OH group). Therefore, there is no intermolecular hydrogen bonding between ether molecules, which makes their boiling points much lower than an alcohol with similar mass. Despite the presence of a small dipole moment, ethers have boiling points that are about the same as alkanes of comparable molar mass. (Table ).
| Condensed Structural Formula | Name | Molar Mass | Boiling Point (°C) | Intermolecular Hydrogen Bonding in Pure Liquid? |
|---|---|---|---|---|
| CH3CH2CH3 | propane | 44 | –42 | no |
| CH3OCH3 | dimethyl ether | 46 | –25 | no |
| CH3CH2OH | ethyl alcohol | 46 | 78 | yes |
| CH3CH2CH2CH2CH3 | pentane | 72 | 36 | no |
| CH3CH2OCH2CH3 | diethyl ether | 74 | 35 | no |
| CH3CH2CH2CH2OH | butyl alcohol | 74 | 117 | yes |
Ether molecules do have an oxygen atom, however, and engage in hydrogen bonding with water molecules. Consequently, an ether has about the same solubility in water as the alcohol that is isomeric with it. For example, dimethyl ether and ethanol (both having the molecular formula C2H6O) are completely soluble in water, whereas diethyl ether and 1-butanol (both C4H10O) are barely soluble in water (8 g/100 mL of water).
Peroxide Formation
Many ethers can react with oxygen to form explosive peroxide compounds n a free radical process called autoxidation. For this reason ethers should not be stored for long periods of time and should not be stored in glass bottles. The danger is particularly acute when ether solutions are distilled to near dryness. The hydroperoxides can become more concentrated during a distillation because they tend to have a slightly higher boiling point than the corresponding ether. Before performing an ether distillation great care should be taken to test for the presence of peroxides.
Naming Ethers
When no other functional group is present, simple ethers are often given common functional class names. Both alkyl groups attached to the oxygen atom are named as substituents (in alphabetical order) and then the word ether is added. The common names for alkyl substituents discussed in Section 3.3 are often used.
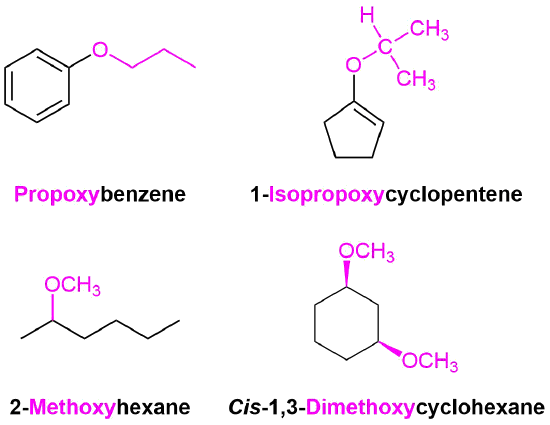
IUPAC nomenclature for ethers should be used for complicated ethers, compounds with more than one ether linkage, and compounds where other functional groups are present with an ether. In these cases, an RO group of the ether is named as an alkoxy substituent. Common alkoxy substituents are given names derived from their alkyl component. The suffix -yl is replaced with -oxy. (Table ):
| Alkyl Group | Name | Alkoxy Group | Name | |
|---|---|---|---|---|
| CH3– | Methyl | CH3O– | Methoxy | |
| CH3CH2– | Ethyl | CH3CH2O– | Ethoxy | |
| (CH3)2CH– | Isopropyl | (CH3)2CHO– | Isopropoxy | |
| (CH3)3C– | tert-Butyl | (CH3)3CO– | tert-Butoxy | |
| C6H5– | Phenyl | C6H5O– | Phenoxy |
Cyclic Ethers
Cyclic ethers are a type of heterocycle with one or more oxygens located in the ring. Many cyclic ethers have common names and are often used as solvents due to their inert nature. These ring structures are also found in many biological molecules such as sugars and DNA. The rings are numbered so that an oxygen gets position 1.

Name the following ethers:
b)
c)
d)
e)
f)
g)
- Answers
-
a) 3-Isopropoxypentane
b) 1,3-Dimethoxybenzene
c) 2-Methyltetrahydropyran
d) Cyclopentyl ethyl ether
e) 4-Bromo-1-ethoxybenzene
f) Dicyclohexyl ether
g) 4-Butoxycyclohexene

