18.7: Crown Ethers
- Page ID
- 36371
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- write the normally accepted name for a crown ether, given its structure.
- draw the structure of a crown ether, given its normally accepted name.
- describe, briefly, the uses of crown ethers.
Make certain that you can define, and use in context, the key term below.
- crown ether
A “crown ether ” is a cyclic ether containing several (i.e., 4, 5, 6 or more) oxygen atoms. As we have indicated in the objectives above, a detailed knowledge of these compounds is not required in this course.
Crown Ethers
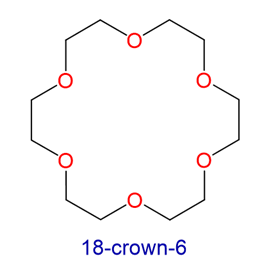
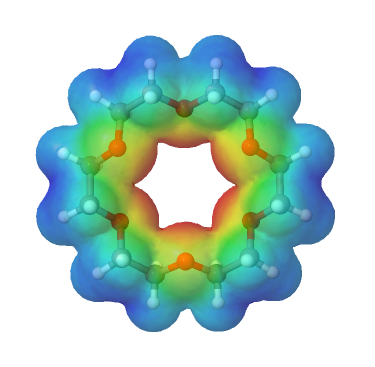
Crown ethers are cyclic polyethers with four or more oxygen atoms each separated by two or three carbon atoms. Crown ethers have the general formula of (OCH2CH2)n or (OCH2CH2CH2)n and are named using both the total number of atoms in the ring and the number of oxygen atoms. Thus 18-crown-6 is an 18-membered ring with six oxygen atoms. All crown ethers have a cavity in the center this is lined with oxygen atoms and can accommodate a alkali metal ion, such as K+. The cation is stabilized by interacting with lone pairs of electrons on the surrounding oxygen atoms. The negative character of center cavity of 18-crown-6 can be seen by looking at its electrostatic potential map shown below. The presence of high electron density is shown by a red color.
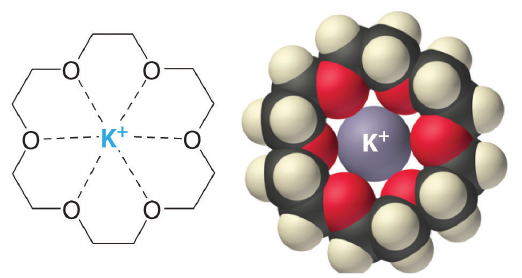
Crown ethers are useful for dissolving ionic substances in organic solvents, such as KMnO4 dissolving in toluene, by sequestering the cations inside a hydrophilic cavity, whereas the outer shell, consisting of C–H bonds, is hydrophobic.
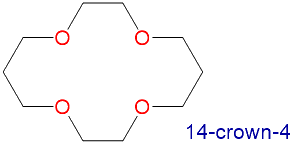
The availability of crown ethers with cavities of different sizes allows specific cations to be solvated with a high degree of selectivity. Crown ethers prefer to bind alkali metal cations with sizes that match that of their binding cavity. For instance, as shown in Table \(\PageIndex{1}\), 14-crown-4 preferentially binds to Li+, 15-crown-5 preferentially binds to Na+, 18-crown-6 preferentially binds to K+, and 21-crown-7 preferentially binds to Cs+.
| Crown Ether | Cavity Diameter (A˚) | Preferred Cation | Cation Diameter (A˚) |
|---|---|---|---|
 |
1.2-1.5 | Li+ | 1.36 |
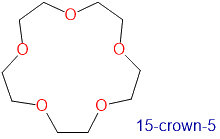 |
1.7-2.2 | Na+ | 1.94 |
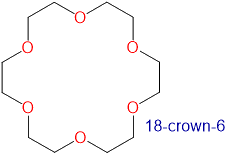 |
2.6-3.2 | K+ | 2.66 |
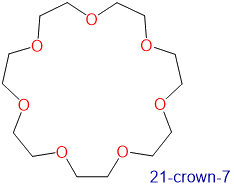 |
3.4-4.3 | Cs+ | 3.34 |
Cryptands
Cryptands (from the Greek kryptós, meaning “hidden”) are variations of crown ethers comprised of two nitrogens connected by three polyether strands. A common nomenclature is used where the numbers preceding the word cryptan indicate the number of oxygen atoms in each strand of the molecule.
Like crown ethers, cryptands are compounds that contains a central cavity that can completely surround a cation with lone pair electrons from oxygen and nitrogen atoms. Also, cryptands can be used to prepare solutions of ionic compounds in solvents that are otherwise too nonpolar to dissolve them. Similar to crown ethers, cryptands prefer to bind with alkali metal cations whose diameter matches the size of their binding cavity.
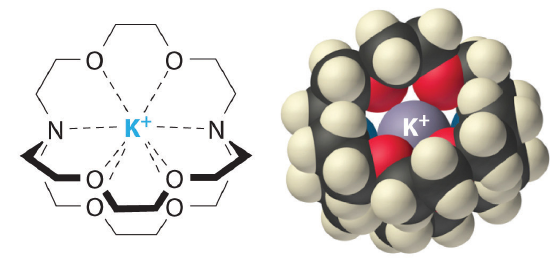
Cryptand ligands also preferentially bind alkali metal ions with sizes that match the size of their binding cavities. The structure and approximate cavity sizes of several cryptands are shown above. Use the information in Table \(\PageIndex{1}\) to predict which alkali metal ion each cryptand will preferentially bind.
- Answer
-
The cryptands might be expected to selectively bind the largest ion which fits within the cavity. Predicted selectivity of crown ethers for alkali metal cations based on the hypothesis that they will selectively bind the largest ion which fits their binding cavity are listed below.
Cryptand Cavity Diameter of (Å) Preferred Cation Cation Diameter (Å) 2.1.1-cryptand 1.60 Li+ 1.36 2.2.1-cryptand 2.20 Na+ 1.96 2.2.2-cryptand 2.80 K+ 2.66 3.2.2-cryptand 3.60 Cs+ 3.34

