17.5: Alcohols from Carbonyl Compounds - Grignard Reagents
- Page ID
- 36349
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- write an equation to illustrate the formation of a Grignard reagent.
- write a general equation to represent the reaction of an aldehyde or ketone with a Grignard reagent.
- write the detailed mechanism for the reaction of an aldehyde or ketone with a Grignard reagent.
- identify the product formed from the reaction of a given aldehyde or ketone with a given Grignard reagent.
- identify the carbonyl compound, the Grignard reagent, or both, needed to prepare a given alcohol.
- write the equation to describe the reaction of an ester with a Grignard reagent.
- identify the product formed from the reaction of a given ester with a given Grignard reagent.
- discuss the limitations of Grignard reagent formation, and determine whether a given compound can be used to form such a reagent.
Before you begin this section, you may wish to review Section 10.8 which discusses the formation of Grignard reagents. Link to section 10.8
Grignard reagents are among the most frequently used reagents in organic synthesis. They react with a wide variety of substrates; however, in this section, we are concerned only with those reactions that produce alcohols. Notice that in a reaction involving a Grignard reagent, not only does the functional group get changed, but the number of carbon atoms present also changes. This fact provides us with a useful method for ascending a homologous series. For example:
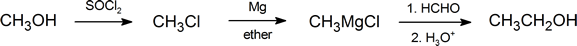
One important route for producing an alcohol from a Grignard reagent has been omitted from the discussion in the reading. It involves the reaction of the Grignard reagent with ethylene oxide to produce a primary alcohol containing two more carbon atoms than the original Grignard reagent.
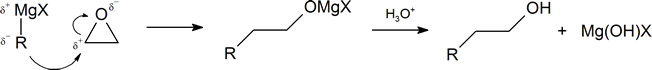
As mentioned in the reading, both organolithium and Grignard reagents are good nucleophiles. They also act as strong bases in the presence of acidic protons such as −CO2H, −OH, −SH, −NH and terminal alkyne groups. Not only do acidic protons interfere with the nucleophilic attack on the carbonyl of these organometallic reagents, if the starting materials possess any acidic protons, reagents cannot be generated in the first place. They are also the reason these reactions must be carried out in a water-free environment.
Another limitation of preparing Grignard and organolithium reagents is that they cannot already contain a carbonyl group (or other electrophilic multiple bonds like C=N, nitriles, N=O, S=O) because it would simply react with itself.
A summary of the methods used to prepare alcohols from Grignard reagents is provided below.
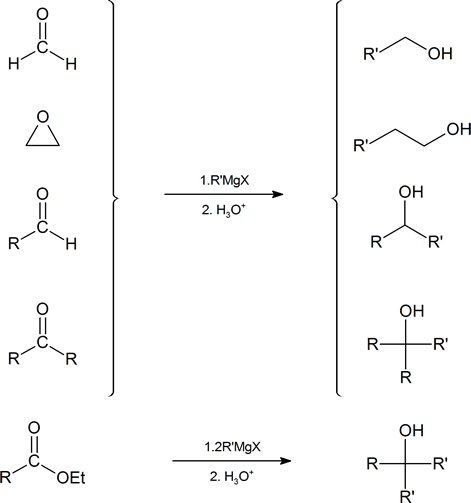
Formation of Grignard Reagents
Grignard reagents (RMgX) can be prepared through the reaction of halogens with magnesium metal (Section 10-6). Grignard reagents are a source of carbanion nucleophiles (R:- +MgX) which add to carbonyl compounds to yield alcohols. Ethyl ether or THF are essential for Grignard reagent formation. Lone pair electrons from two ether molecules form a complex with the magnesium in the Grignard reagent. This complex helps stabilize the organometallic and increases its ability to react.
Reaction of Grignard Reagents with Carbonyls
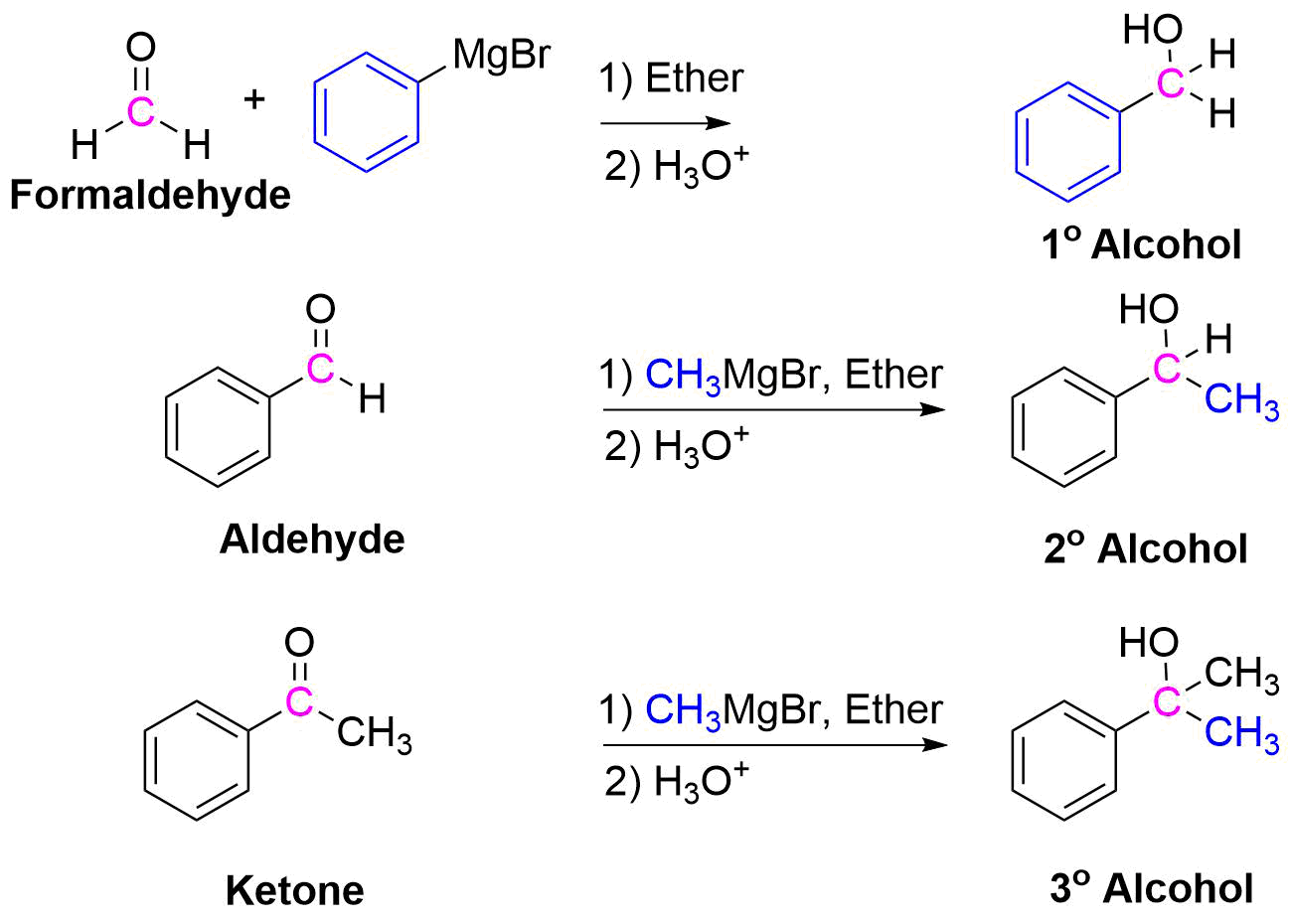
Because organometallic reagents react as their corresponding carbanion, they are excellent nucleophiles. Aldehydes, ketones, and other carbonyl containing compounds will undergo nucleophilic addition with Grignard reagents. The nucleophilic carbon in the organometallic reagents forms a C-C single bond with the electrophilic carbonyl carbon. An alkoxide ion intermediate is formed which becomes an alcohol with subsequent protonation by an acid. The type of alcohol produced depends on the number of alkyl substituents attached to the electrophilic carbonyl carbon. Reacting a Grignard reagent with formaldehyde (H2C=O) produces 1o alcohols, aldehydes produce 2o alcohols, and ketones produce 3o alcohols. These reactions will be discussed in greater detail in Section 19.7.
General Reaction
Addition to Formaldehyde gives 1o Alcohols
Addition to Aldehydes gives 2o Alcohols
Addition to Ketones gives 3o Alcohols
Predicting the Product of the Addition of Grignard Reagent to Carbonyl
During the reaction, the C=O double bond in the reactant forms a C-O single bond in the product. The breaking of the C=O double bond allows for the formation of two single bonds in the product. One will be attached to the oxygen and one to the carbon which was originally in the carbonyl. The carbon will gain whatever R group was contained in the Grignard reagent and the oxygen will gain a hydrogen.
Mechanism for the Addition of Grignard Reagents to Carbonyls
The mechanism starts with the formation of a acid-base complex between +MgX and the carbonyl oxygen. The +MgBr of the Grignard reagent acts as a Lewis acid and accepts a set of lone pair electrons from the carbonyl oxygen. This gives the oxygen a positive charge which correspondingly increases the partial positive charge on the carbonyl carbon increasing its susceptibility to nucleophilic attack.
Step 1: Lewis acid-base formation
Step 2: Nucleophilic attack
The carbanion nucleophile from the Grignard reagent adds to the electrophilic carbon of the acid-base complex forming a C-C bond. The two electrons of the C=O are pushed toward the carbonyl oxygen atom forming a tetrahedral Magnesium alkoxide intermediate.
Step 3: Protonation
The alkoxide intermediate is converted to an alcohol through addition of a acidic aqueous solution. The +MgX ion is also converted to HOMgX.'
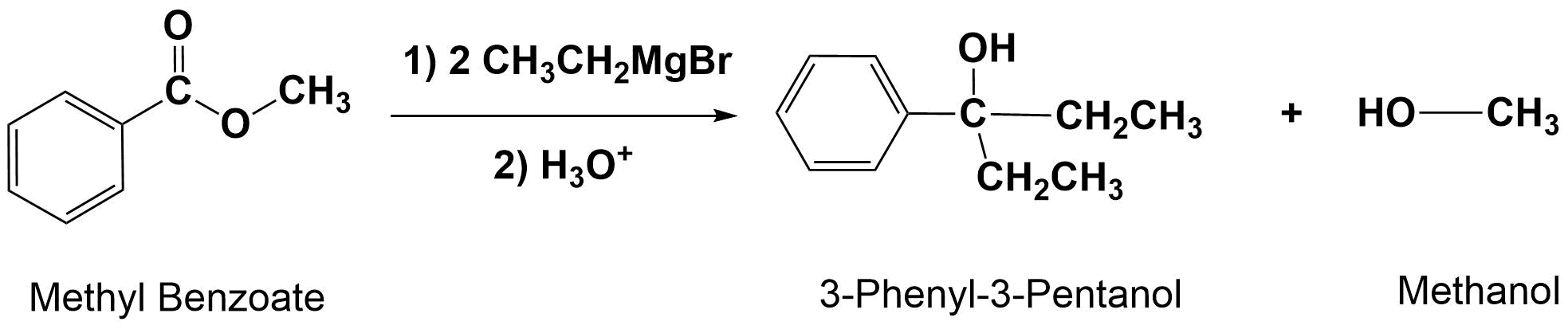
Grignard Reagents Convert Esters to 3o alcohols
With esters, after the first Grignard reaction, the carbonyl reforms creating a ketone which can then react with a second molecule of the Grignard. In effect, the Grignard reagent adds twice. This reaction will be discussed in greater detail in Section 21.6.
General Reaction
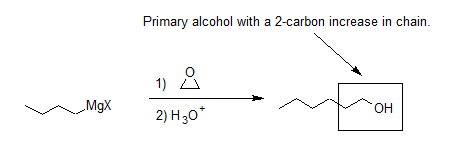
Grignard Reagents Convert Epoxides to 1o alcohols
Another important route for producing an alcohol from a Grignard reagent involves the reaction of the Grignard reagent with ethylene oxide to produce a primary alcohol containing two more carbon atoms than the original Grignard reagent. This reaction will be discussed in greater detail in Section 18.6.
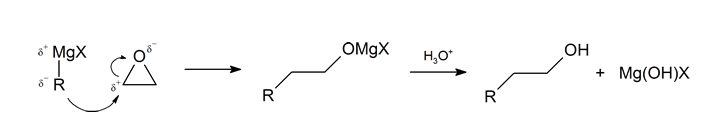
The first step of the mechanism is shown below. With the second step following the protonation step common to the other reaction pathways studied in this section.
Limitation of Organometallic Reagents
Grignard and organolithium reagents are powerful bases. Because of this they cannot be prepared using halogen compounds which contain an additional functional group that has acidic hydrogens. If there are acidic hydrogens present, the organometallic reagent will act as a base and deprotonate the acidic hydrogen rather than act as a nucleophile and attack the carbonyl. A partial list of functional groups which cannot be used are: alcohols (ROH), carboxylic acids (RCO2H), thiols (RSH), and terminal alkynes (RCCH). Additionally, amides (RCONH2) and amines (RNH2) that have NH bonds cannot be used with organometallic reagents.
Planning an Alcohol Synthesis Using a Grignard Reaction
The nucleophilic addition of a Grignard reagent to a carbonyl is a powerful tool in organic synthesis because if forms a C-C bond. Also, there is often more than one way to make a given target molecule. Primary alcohols have one C-C bond which can retrosynthetically cleaved. Secondary alcohols have two and tertiary alcohols have three.
What reagents are required to make the following molecule using a Grignard Reaction?
- Answer
- Analysis: Because the target molecule is a 1o alcohol there is only one C-C bond which can be cleaved to generate possible starting materials. The only possible reagents which would provide the target molecule would be formaldehyde and phenylmagnesium bromide.
What reagents are required to make the following molecule using a Grignard Reaction?
- Answer
-
Analysis: Because the target molecule is an asymmetrical 2o alcohol there are two different C-C bond cleavage points. Each of these will provide a unique set of reagents which should be considered in terms of their reactivity and availability.
Pathway 1)
Pathway 1 synthesis)
Pathway 1 shows that propanal and propylmagnesium bromide can be reacted to create the target molecule.
Pathway 2)
Pathway 2 synthesis)
Pathway 2 shows that butanal and ethylmagnesium bromide can be reacted to create the target molecule.
Exercises
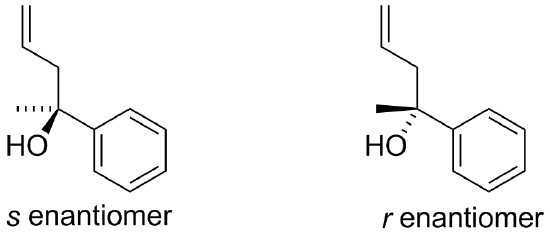
1) If allylmagnesium chloride were added to a solution of the following compound and then worked-up with acid, the product would contain a chiral center. Would the product be a racemic mixture or an enatiomerically pure product? Draw both enantiomers.
2) What combination of carbonyl compound and grignard (use MgBr) reagent would yield the following alcohols (after workup)?
a) 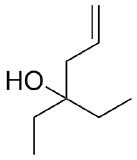 b)
b) 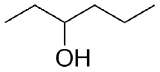 c)
c) 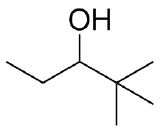 d)
d)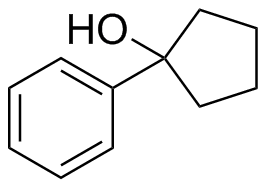
3) If the following compounds were reacted with methylmagnesium bromide what would be the products?
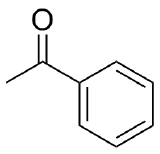
a) Cyclohexanone b) Acetophenone c) 2-Hexanone
- Answer
-
1) The result would be a racemic mixture of the following.
2)
c)
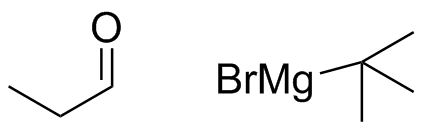 d)
d) 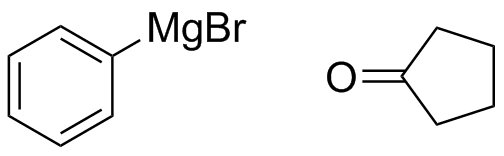
3)
a)
b)
c)
If allylmagnesium chloride were added to a solution of the following compound and then worked-up with acid, the product would contain a chiral center. Would the product be a racemic mixture or an enatiomerically pure product? Draw both enantiomers.
- Answer
-
The result would be a racemic mixture of the following.
What combination of carbonyl compound and grignard (use MgBr) reagent would yield the following alcohols (after workup)?
- Answer
-
Fill in the blanks of the following reaction scheme.
- Answer
-

