16.4: Substituent Effects in Substituted Aromatic Rings
- Page ID
- 31576
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- describe the two ways in which a substituent influences the electrophilic substitution of a monosubstituted aromatic compound.
- classify each of the following substituents as being either activating or deactivating with respect to electrophilic aromatic substitution: \(\ce{\sf{-NH2}}\), \(\ce{\sf{-OH}}\), \(\ce{\sf{-NHR}}\), \(\ce{\sf{-NR2}}\), \(\ce{\sf{-OR}}\), \(\ce{\sf{-NHCOR}}\), alkyl (R), phenyl, \(\ce{\sf{R3N+}}\), \(\ce{\sf{-NO2}}\), \(\ce{\sf{-CN}}\), \(\ce{\sf{-COR}}\), \(\ce{\sf{-CO2H}}\), \(\ce{\sf{-CO2R}}\), \(\ce{\sf{-CHO}}\), halogens.
- list a given series of substituents (selected from those given in Objective 2) in order of increasing or decreasing ability to activate or deactivate an aromatic ring with respect to electrophilic substitution.
- explain, in general terms, the factors that determine whether a given substituent will activate or deactivate an aromatic ring with respect to electrophilic substitution.
- list a given series of aromatic compounds in order of increasing or decreasing reactivity with respect to electrophilic substitution.
- explain the inductive effects displayed by substituents such as nitro, carboxyl, alkyl and the halogens during electrophilic aromatic substitution reactions.
- explain the resonance effects displayed by substituents such as nitro, carbonyl-containing, hydroxy, alkoxy and amino groups during electrophilic aromatic substitution reactions.
Make certain that you can define, and use in context, the key terms below.
- inductive effect
- resonance effect
On reading Objective 2 students may exclaim “How am I ever going to memorize all of this!”—or words to that effect. The answer is that if you are trying to memorize such things, you are taking the wrong approach to organic chemistry. What you should be doing is trying to understand the factors that determine whether a given substituent will activate or deactivate a benzene ring with respect to electrophilic substitution.
You may wish to review earlier material on to the inductive effect. If so, refer to Sections 2.1, 7.9 (paying particular attention to the “Study Notes”) and 14.5.
Note that one argument sometimes used to explain the ability of alkyl groups to donate electrons inductively to an aromatic ring is that sp2‑hybridized carbon atoms are more electronegative than sp3‑hybridized carbon atoms. Thus, a sigma bond between sp2- and sp3‑carbon is slightly polarized, as follows:

When substituted benzene compounds undergo electrophilic substitution reactions of the kind discussed above, two related features must be considered:
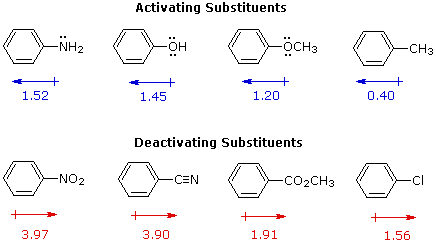
I. The first is the relative reactivity of the compound compared with benzene itself. Experiments have shown that substituents on a benzene ring can influence reactivity in a profound manner. For example, a hydroxy or methoxy substituent increases the rate of electrophilic substitution about ten thousand fold, as illustrated by the case of anisole in the virtual demonstration (above). In contrast, a nitro substituent decreases the ring's reactivity by roughly a million. This activation or deactivation of the benzene ring toward electrophilic substitution may be correlated with the electron donating or electron withdrawing influence of the substituents, as measured by molecular dipole moments. In the following diagram we see that electron donating substituents (blue dipoles) activate the benzene ring toward electrophilic attack, and electron withdrawing substituents (red dipoles) deactivate the ring (make it less reactive to electrophilic attack).
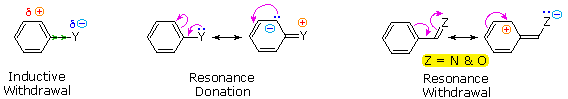
The influence a substituent exerts on the reactivity of a benzene ring may be explained by the interaction of two effects:
The first is the inductive effect of the substituent. Most elements other than metals and carbon have a significantly greater electronegativity than hydrogen. Consequently, substituents in which nitrogen, oxygen and halogen atoms form sigma-bonds to the aromatic ring exert an inductive electron withdrawal, which deactivates the ring (left-hand diagram below).
The second effect is the result of conjugation of a substituent function with the aromatic ring. This conjugative interaction facilitates electron pair donation or withdrawal, to or from the benzene ring, in a manner different from the inductive shift. If the atom bonded to the ring has one or more non-bonding valence shell electron pairs, as do nitrogen, oxygen and the halogens, electrons may flow into the aromatic ring by p-π conjugation (resonance), as in the middle diagram. Finally, polar double and triple bonds conjugated with the benzene ring may withdraw electrons, as in the right-hand diagram. Note that in the resonance examples all the contributors are not shown. In both cases the charge distribution in the benzene ring is greatest at sites ortho and para to the substituent.
In the case of the nitrogen and oxygen activating groups displayed in the top row of the previous diagram, electron donation by resonance dominates the inductive effect and these compounds show exceptional reactivity in electrophilic substitution reactions. Although halogen atoms have non-bonding valence electron pairs that participate in p-π conjugation, their strong inductive effect predominates, and compounds such as chlorobenzene are less reactive than benzene. The three examples on the left of the bottom row (in the same diagram) are examples of electron withdrawal by conjugation to polar double or triple bonds, and in these cases the inductive effect further enhances the deactivation of the benzene ring. Alkyl substituents such as methyl increase the nucleophilicity of aromatic rings in the same fashion as they act on double bonds.
Exercises
Draw the resonance structures for benzaldehyde to show the electron-withdrawing group.
- Answer
-
Draw the resonance structures for methoxybenzene to show the electron-donating group.
- Answer
-

