11.S: Reactions of Alkyl Halides - Nucleophilic Substitutions and Eliminations (Summary)
- Page ID
- 210489
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Concepts & Vocabulary
- Alkyl halides react as electrophiles and undergo nucleophilic substitution and elimination reactions.
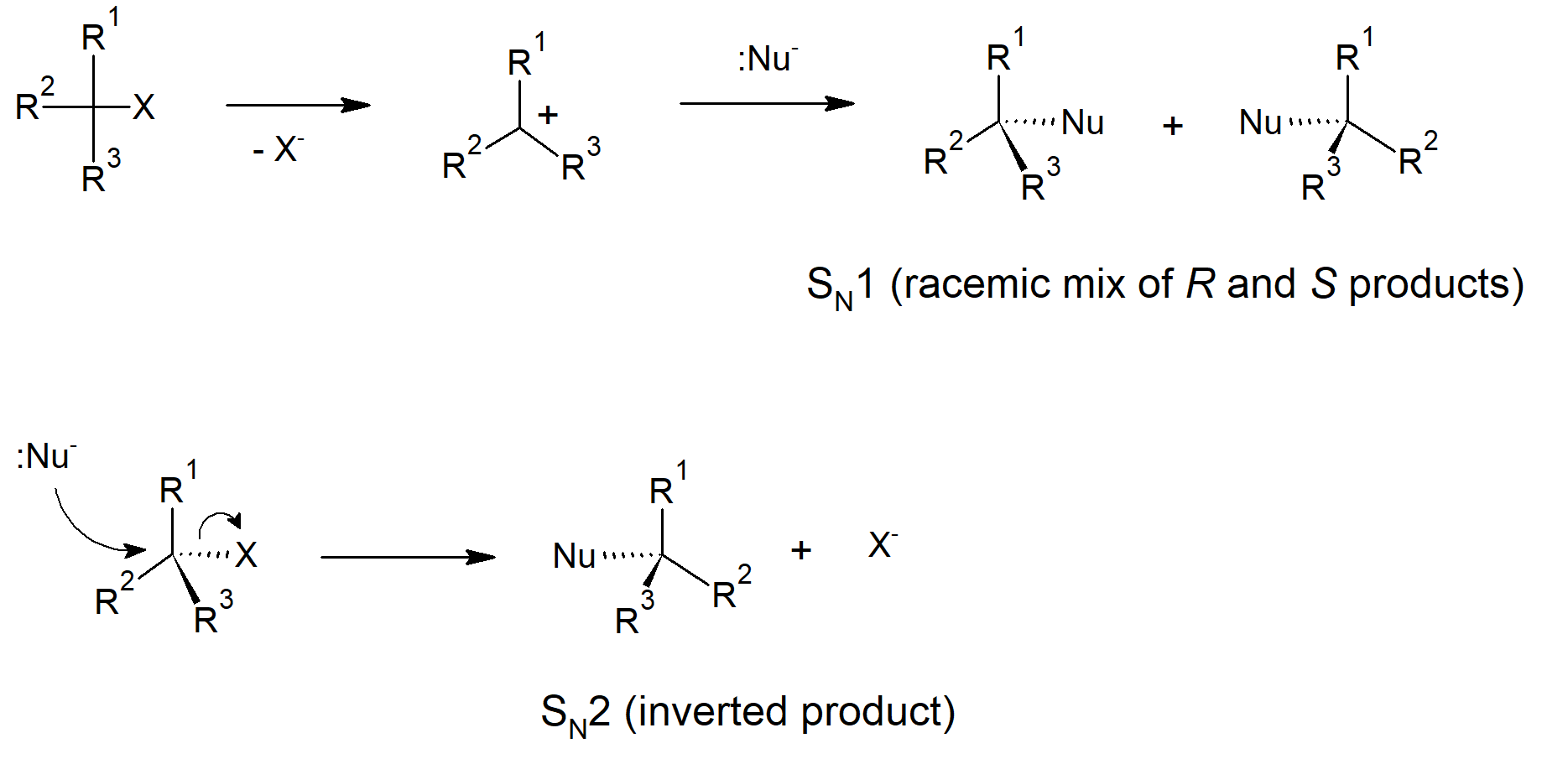
11.2 The Discovery of Nucleophilic Substitution Reactions
- Some nucleophilic substitution reactions invert stereochemistry at the reactive carbon.
- Reaction steps with two molecules involved in the rate determining step are called bimolecular.
- A substitution mechanism that has the nucleophile entering at the same time the leaving group leaves, in a concerted step, is called SN2 - substitution nucleophilic bimolecular.
- Concerted substitution mechanisms (SN2) occur via backside attack, which causes inversion of the carbon where the reaction occurs.
- Rates of SN2 reactions depend on concentration of nucleophile and alkyl halide.
11.4 Characteristics of the SN2 Reaction
- SN2 reactions are concerted.
- Sterically hindered substrates reduce SN2 reaction rate.
- A transition state in a reaction mechanism is the highest energy point on a pathway from reactants to an intermediate or products.
- Larger groups (such as alkyl vs. hydrogen) cause greater steric repulsion in SN2 transition states, reducing rates of SN2 reactions.
- Groups that have electron-rich atoms are typically good nucleophiles.
- In general, stronger bases are better nucleophiles.
- Polar aprotic solvents increase rates of SN2 reactions.
- Polar protic solvents decrease rates of SN2 reactions.
- As basicity of leaving groups decreases, their ability to leave increases.
- A substitution mechanism that occurs with the leaving group leaving in the first step, creating a carbocation intermediate, followed by the nucleophile entering is called SN1 - substitution nucleophilic unimolecular.
- SN1 reactions occur through a stepwise mechanism.
- The first step (dissociation) of an SN1 mechanism is rate limiting.
- In SN1 reactions the nucleophile is not involved in the rate limiting step, therefore nucleophile strength or concentration do not affect the rate.
- The intermediate for SN1 mechanisms contains a planar carbocation. The nucleophile can then enter from either side of the molecule giving racemic products with no additional stereocenters in the molecule.
11.6 Characteristics of the SN1 Reaction
- Polar solvents increase rates of SN1 reactions.
- Better leaving groups increase rates of SN1 and SN2 reactions.
- Predicting whether a reaction will follow an SN1 or SN2 mechanism requires analysis of:
- Electrophile - primary favor SN2, tertiary (and allyl or benzyl) favor SN1, secondary depends on other factors
- Nucleophile - strong favor SN2, weak favor SN1
- Solvent - polar aprotic favor SN2, polar protic favor SN1
11.7 Biological Substitution Reactions
- When biological substitution reactions occur, the electrophiles are often different though the mechanisms are primarily the same.
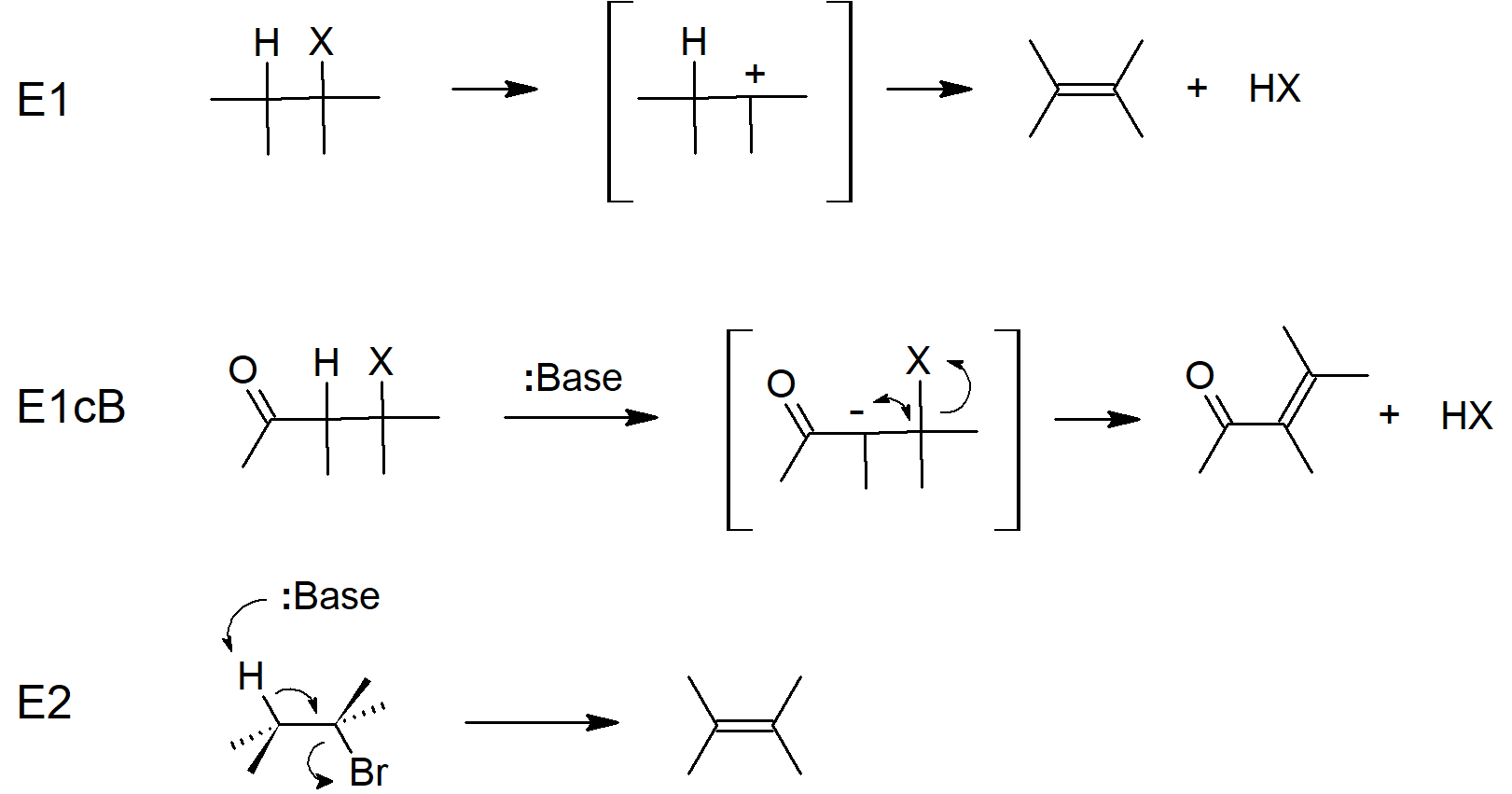
11.8 Elimination Reactions - Zaitsev's Rule
- The major product of Elimination reactions is the product with the more substituted double bond. This is known as Zaitsev's rule.
11.9 The E2 Reaction and Deuterium Isotope Effect
- The E2 mechanism is concerted with the base removing a proton and the leaving group leaving at the same time.
- Since E2 mechanisms are concerted, both the base and the electrophile are present in the rate equation.
- E2 reactions require strong bases and polar aprotic solvents.
- Kinetic Isotope Effects can provide evidence for E2 mechanisms since they can show when breaking of the C-H bond is part of the rate-determining step.
11.10 The E2 Reaction and Cyclohexane Conformation
- E2 reactions of cyclic structures show necessity for anti orientation of the proton being removed and the leaving group.
11.11 The E1 and E1cB Reactions
- E1 mechanisms begin with a leaving group leaving which forms a carbocation intermediate, which is then deprotonated in a second step.
- E1 mechanisms are step-wise.
- More substituted electrophiles are more reactive in E1 reactions.
- Zaitsev products are preferred, similarly to E2 reactions.
- E1 and SN1 proceed via the same carbocation intermediate and the same rate-determining step so typically happen concurrently.
- E1cB reactions begin with deprotonation (usually resulting in a resonance stabilized carbanion), followed by loss of the leaving group in the second step.
11.12 Biological Elimination Reactions
- There are many important examples of biological elimination reactions.
11.13 A Summary of Reactivity - SN1, SN2, E1, E1CcB, and E2
Skills to Master
- Skill 11.1 Draw SN1/SN2 mechanisms showing appropriate stereochemistry.
- Skill 11.2 Explain when SN1/SN2 mechanisms are likely to occur.
- Skill 11.3 Describe/draw the intermediate for an SN1 mechanism and transition state(s) for SN1/SN2 mechanisms.
- Skill 11.4 Write out rate laws for SN1/SN2 mechanisms.
- Skill 11.5 Differentiate between which mechanism is more likely between SN1/SN2.
- Skill 11.6 Draw reaction coordinate diagrams for SN1/SN2 mechanisms.
- Skill 11.7 Explain how the electrophile, nucleophile, leaving group, and solvent affect SN1/SN2 mechanisms.
- Skill 11.8 Recognize use of nucleophilic substitution and elimination reactions in biological systems.
- Skill 11.9 Draw E1/E2 mechanisms showing appropriate stereochemistry.
- Skill 11.10 Explain when E1/E2 mechanisms are likely to occur.
- Skill 11.11Describe/draw the intermediate for an E1 mechanism and transition state(s) for E1/E2mechanisms.
- Skill 11.12 Write out rate laws for E1/E2 mechanisms.
- Skill 11.13 Differentiate between which mechanism is more likely between E1/E2.
- Skill 11.14 Draw reaction coordinate diagrams for E1/E2 mechanisms.
- Skill 11.15 Explain how kinetic isotope effects can be used to support or refute a proposed mechanism.
- Skill 11.16 Draw an E1cB mechanism and explain when it is a viable option.
- Skill 11.17 Differentiate between which mechanism is more likely between SN1/SN2 and E1/E2.
Memorization Tasks (MT)
MT 11.1 Memorize the order of good leaving groups.
MT 11.2 Memorize which solvents are polar protic and polar aprotic.
MT 11.3 Memorize the stability order of carbocations.
Summary of Reactions
Nucleophilic Substitutions
Eliminations