23.1: Carbonyl Condensations - The Aldol Reaction
- Page ID
- 36423
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- write a general mechanism for carbonyl condensation reactions.
- write an equation to illustrate the aldol condensation reaction.
- identify the product formed when an aldehyde or ketone having an alpha‑hydrogen atom is treated with base in a protic medium.
- identify the aldehyde or ketone, and other reagents required to produce a given β‑hydroxy carbonyl compound by an aldol reaction.
- determine whether a given aldehyde or ketone will undergo an aldol reaction.
- write the detailed mechanism of the aldol reaction.
- aldol
- aldol reaction
- carbonyl condensation reaction (see Chapter 18 Affix)
It is important that you understand the general mechanism of carbonyl condensation described in this section: once you grasp this mechanism, you will see that all the reactions that follow are very similar.
The aldol reaction is sometimes referred to as the aldol condensation. However, a condensation reaction is often regarded as a reaction in which two molecules join together with the elimination of a molecule of water (or some other compound of low molar mass). Thus, the aldol reaction described here is not a true condensation; the true aldol condensation is described later, in Section 23.3. It is perhaps unfortunate that the reactions discussed in this unit are all described as condensation reactions whether or not water is eliminated.
The term “aldol” (from "aldehyde alcohol") is used both to describe the specific compound 3‑hydroxybutanal:

and to describe β‑hydroxy aldehydes in general.
A useful carbon-carbon bond-forming reaction known as the Aldol Reaction is another example of electrophilic substitution at the alpha carbon in enolate anions. This reaction requires the formation of an enolate so at least one of the reactants must have an α-hydrogen. Due to the carbanion like nature of enolates, they can add to carbonyls through nucleophilic addition much like Grignard reagents.
The aldol reaction takes advantage of a carbonyl compound’s ability to undergo both alpha substitution and nucleophilic addition reactions. The fundamental transformation in the aldol reaction is a dimerization of an aldehyde (or ketone) to form a beta-hydroxy aldehyde (or ketone). A C-C bond is formed between the alpha carbon of one reactant molecule and the carbonyl carbon of a second reactant molecule. In the reaction’s product, the formed C-C bond links a carbon in the alpha position and a carbon in the beta position away from the carbonyl.
General Aldol reaction
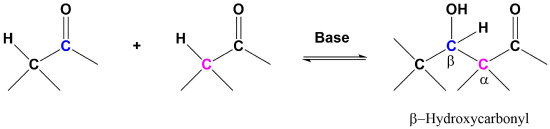
A typical example involves two molecules of acetaldehyde (ethanal) reacting to form beta-hydroxybuteraldehyde (3-hydroxybutanal). This product and other beta-hydroxy aldehydes are generically called “aldols” because they contain both an aldehyde and an alcohol functional group.
An aldol reaction, like many carbonyl addition reactions, is an equilibrium reaction and is reversible. The presence of an equilibrium means weaker bases, such a hydroxides or alkoxides, can be used to perform this reaction. The reaction equilibrium favors the products when aldehydes with little steric hindrance around the carbonyl are used. However, the reaction equilibrium for ketones and sterically hindered aldehydes favors the reactants. To provide good reaction yields when using these reactants, the equilibrium must be pushed towards the products. Typically, this is done by utilizing a method to remove the product as it is formed during the reaction.
Predicting the Product of an Aldol Reaction 
Examples
Mechanism of Aldol Reaction
1) Enolate formation
The reaction starts with a base removing an alpha hydrogen to form a nucleophilic enolate.
2) Nucleophilic attack by the enolate
Through nucleophilic addition, the enolate adds to the electrophilic carbonyl group on a second molecule. As with other nucleophilic addition reaction a tetrahedral alkoxide intermediate is formed.
3) Protonation
Protonation of the alkoxide forms the neutral aldol product and regenerates the base.
Stereochemical Ramifications of the Aldol Reaction
As previously discussed, both nucleophilic addition and alpha-substitution reactions have the possibility of creating chiral carbons. The alpha carbon and the electrophilic carbon of the reactants should be identified in the aldol product to assess their possible chirality. Most aldehydes produce chirality in both of these carbons. Most symmetrical ketones create a chiral carbon from the alpha-carbon of the reactant.
Going from reactants to products simply
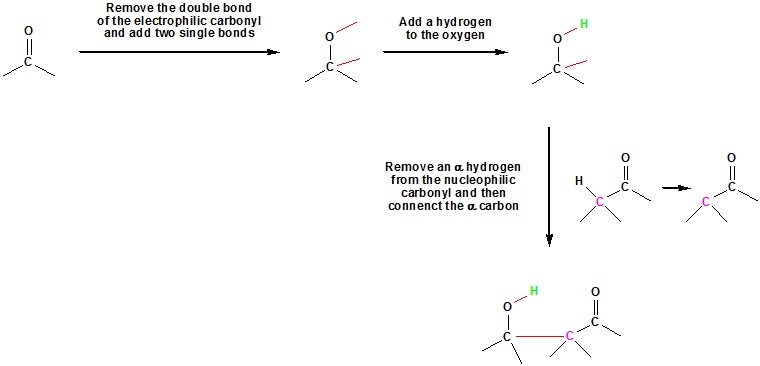
What would be the expect product of the following aldol reaction?
- Answer
-
Analysis:
When considering the product of an aldol reaction it is vital to consider each reactant molecule separately. Also,
Identify electrophilic carbonyl carbon and any alpha hydrogens present.
1) Predict the product of an aldol reaction with the following molecules:
a)
b)
c)
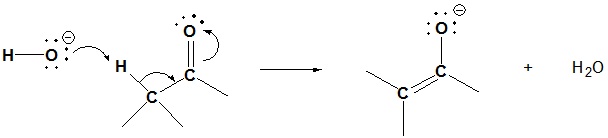
2) Because the aldol reaction is reversible it is possible for a beta-hydroxy carbonyl compound to undergo a retro-aldol reaction. Please draw the mechanism or the based catalyzed retro-aldol reaction shown below.
- Answers
-
1)
a)
b)
c)
2)

