11.9: The E2 Reaction and Cyclohexane Conformation
- Page ID
- 31513
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- identify anti periplanar arrangements of atoms in substituted cyclohexanes.
- determine which cyclohexane conformation will generate a specific anti periplanar arrangement.
Stereochemical Requirements of the E2 Reaction
E2 elimination reactions of certain isomeric cycloalkyl halides show unusual rates and regioselectivity which can provide important supporting evidence that anti-periplanar is the preferred orientation of reactant species in the E2 transition state. Unlike open chain structures, cyclic compounds generally restrict the spatial orientation of ring substituents to relatively few arrangements. The compounds used here all have six-membered rings, so to achieve the anti-periplanar orientation required for the E2 reaction both the halogen and the adjacent hydrogen much assume an axial conformation.
For the compound, 2-methyl-1-chlorocyclohexane, the cis to the trans isomers have distinctly different reaction rates and form different preferred products. For example, trans-2-methyl-1-chlorocyclohexane reacts with alcoholic KOH at a much slower rate than does its cis-isomer. For trans-methyl-1-chlorocyclohexane, to obtains a chair conformation which places the chlorine substituent in the axial orientation required for E2 elimination, the methyl substituents is also forced into an axial position. Having both substituents in the axial position makes this chair conformer of the trans-isomer much less stable and presents an energy barrier that must be overcome for an E2 reaction to occur. When cis-2-methyl-1-chlorocyclohexane obtains a chair conformation which places its chlorine substituent in an axial orientation, the methyl substituent is forced into an equatorial orientation. Having one substituent axial and one equatorial makes this chair conformation of the cis-isomer lower in energy and thus easier to form. Consequently, the E2 reaction rates with the trans-isomer are slower than with the cis-isomer.
Furthermore, the product from E2 elimination of the trans-isomer is 3-methylcyclohexene (not predicted by Zaitsev's rule), whereas the cis-isomer gives the 1-methylcyclohexene as the preferred product (as predicted by Zaitsev's rule). The trans-isomer only has one adjacent hydrogen which can obtain an axial orientation along with the chlorine. The adjacent hydrogen which would lead to a 1-methylcyclohexene product cannot obtain the diaxial, anti-periplanar orientation with chlorine so an E2 reaction cannot occur. This makes 3-methylcyclohexene the preferred E2 elimination product of the trans-isomer showing that the anti-periplanar orientation requirement of E2 reactions is more important in determining products for this reaction than Zaitsev's rule.
In the cis-isomer the smaller chlorine atom assumes an axial position in the more stable chair conformation, and here there are two adjacent axial hydrogens. Removing one hydrogen forms the product, 3-methylcyclohexene, while removing the other hydrogen forms 1-methylcyclohexene. Here Zaitsev's rule determines that the more substituted alkene, 1-methylcyclohexene, is the preferred product.
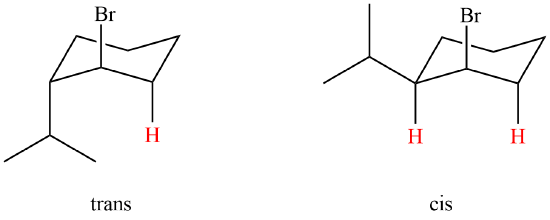
Which isomer would be expected to undergo E2 elimination quicker cis or trans-1-bromo-4-tert-butylcyclohexane? Explain your answer. In the case of the 1-bromo-4-tert-butylcyclohexane isomers, the tert-butyl group is so large that it will always assume an equatorial orientation, leaving the bromine to be axial in the cis-isomer and equatorial in the trans-isomer. Because of symmetry, the two axial adjacent hydrogens in the cis-isomer react equally with base, resulting in rapid elimination to the same alkene (actually a racemic mixture). This reflects the fixed anti orientation of these hydrogens to the bromine atom. To assume a conformation having an axial bromine, the trans-isomer must tolerate serious crowding distortions. Such conformers are therefore present in extremely low concentration, and the rate of elimination is very slow. Indeed, substitution by hydroxide anion predominates in this situation. Which of the following compounds will react faster in an E2 reaction; trans-1-bromo-2-isopropylcyclohexane or cis-1-bromo-2-isopropylcyclohexane? The cis isomer will react faster than the trans. The cis isomer has two possible perpendicular hydrogen in which it can eliminate from.