5.8: More Complex Spin-Spin Splitting Patterns
- Page ID
- 432196
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
Objectives
After completing this section, you should be able to
- explain how multiple coupling can give rise to complex-looking 1H NMR spectra.
- predict the splitting pattern expected in the 1H NMR spectrum of an organic compound in which multiple coupling is possible.
- interpret 1H NMR spectra in which multiple coupling is evident.
Study Notes
Another effect that can complicate a spectrum is the “closeness” of signals. If signals accidently overlap they can be difficult to identify. You can try this yourself by drawing a tree diagram. Keep this point in mind when interpreting real 1H NMR spectra.
Also, when multiplets are well separated, they form patterns. However, when multiplets approach each other in the spectrum they sometimes become distorted. Usually, the inner peaks become larger than the outer peaks. Note the following examples:
Complex coupling
In all of the examples of spin-spin coupling that we have seen so far, the observed splitting has resulted from the coupling of one set of hydrogens to just one neighboring set of hydrogens. What happens if there are hydrogens on both sides?
Similar Coupling Constants
When a proton is coupled to two different neighboring proton sets with identical or very close coupling constants, the splitting pattern that emerges often appears to follow the simple `n + 1 rule` of non-complex splitting. Looking at 1,1,3-trichloropropane below, Ha and Hc only have Hb as neighbors. Remember, splitting occurs primarily between hydrogens that are separated by three bonds. This means that Ha would be a triplet as would Hc. However, Hb has two sets of neighboring protons (Ha and Hc). Each follows the simple spin-spin coupling patterns, since they each just have one neighboring set of hydrogens (Hb). However with Hb, it has neighboring hydrogens on both sides. There are two Ha neighbors and there is one Hc neighbor. It turns out that since the types of interveneing bonds between Ha and Hb are the same as Hb and Hc, the coupling constants will be very similar. Ha and Hc are not equivalent (their chemical shifts are different), but it turns out that 3Jab is very close to 3Jbc.
If we perform a splitting diagram analysis for Hb, we see that, due to the overlap of sub-peaks, the signal appears to be a quartet, and for all intents and purposes follows the n + 1 rule.
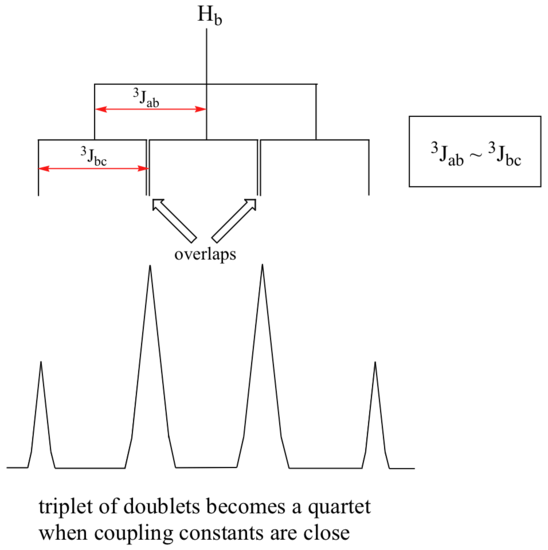
Therefore, we can still use the n + 1 rule when the coupling constants are similar and making it easier for organic chemists.
Different Coupling Constants
When a set of hydrogens is coupled to two or more sets of nonequivalent neighbors, the result is a phenomenon called complex coupling. A good illustration is provided by the 1H-NMR spectrum of methyl acrylate:
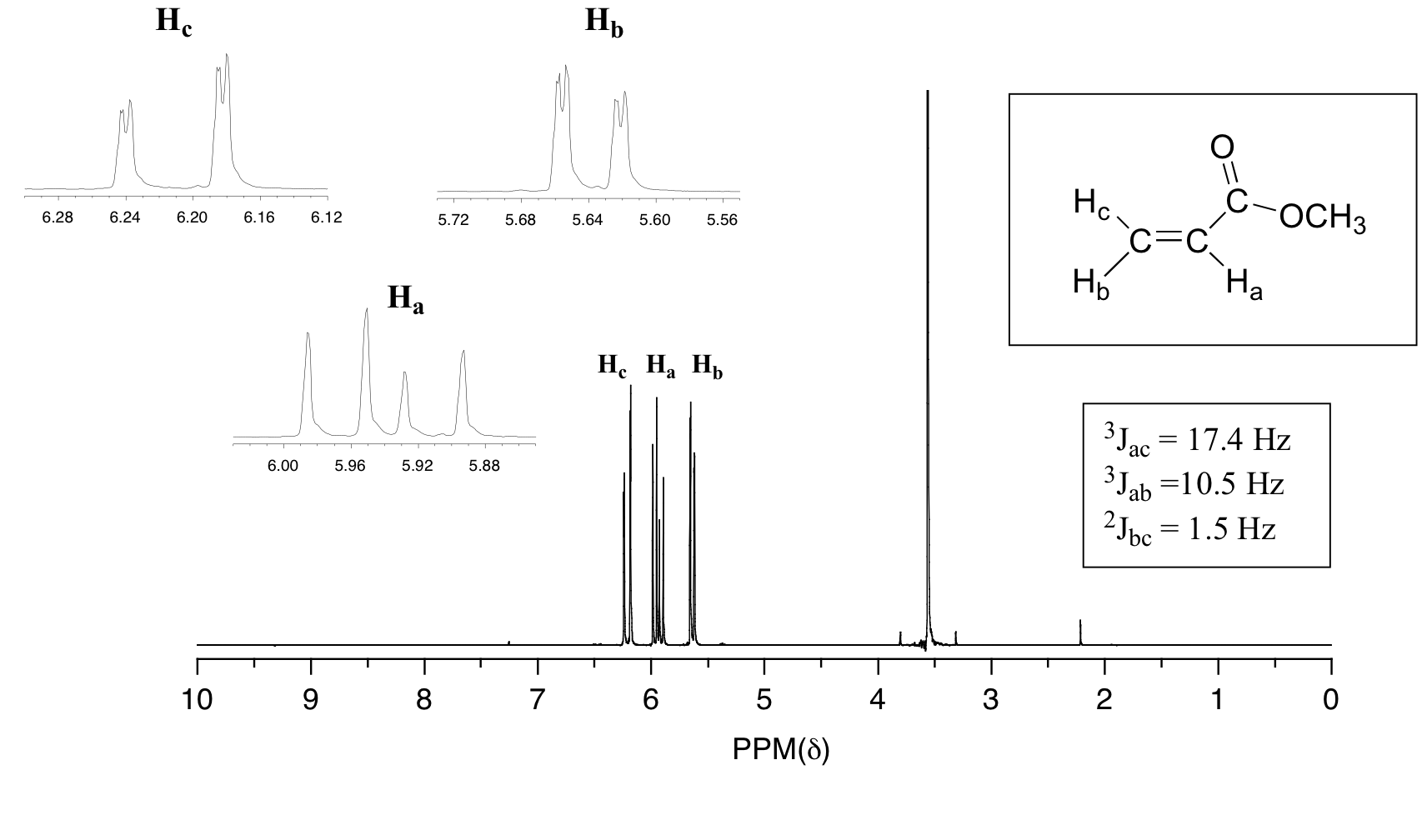
First, let's first consider the Hc signal, which is centered at 6.21 ppm. Here is a closer look:
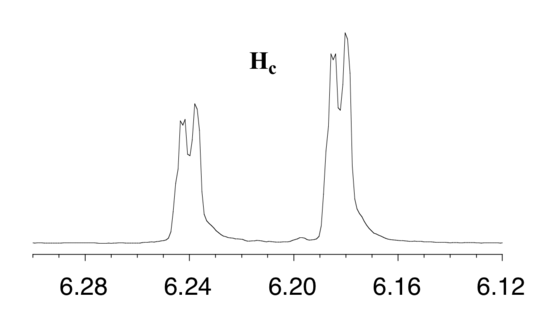
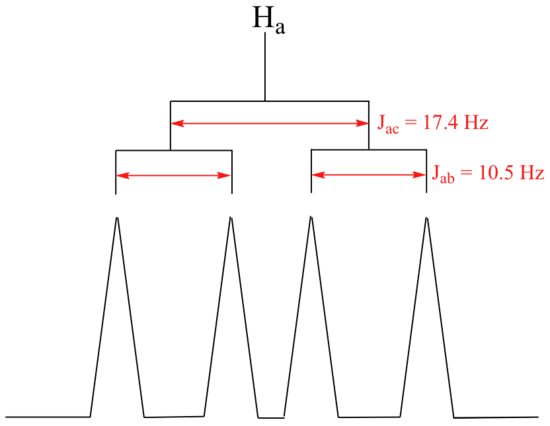
With this enlargement, it becomes evident that the Hc signal is actually composed of four sub-peaks. Why is this? Hc is coupled to both Ha and Hb , but with two different coupling constants. Once again, a splitting diagram (or tree diagram) can help us to understand what we are seeing. Ha is trans to Hc across the double bond, and splits the Hc signal into a doublet with a coupling constant of 3Jac = 17.4 Hz. In addition, each of these Hc doublet sub-peaks is split again by Hb (geminal coupling) into two more doublets, each with a much smaller coupling constant of 2Jbc = 1.5 Hz.
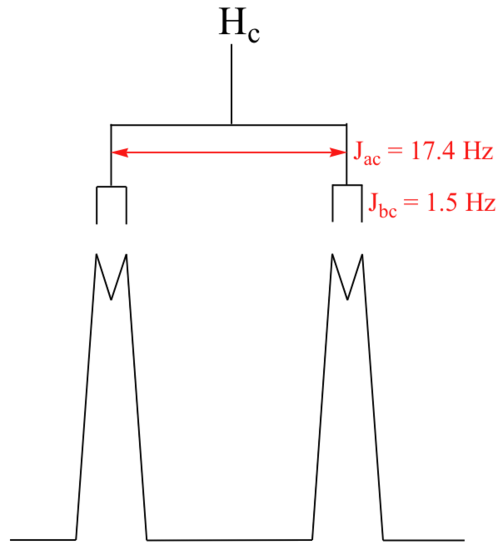
The result of this `double splitting` is a pattern referred to as a doublet of doublets, abbreviated dd.
The signal for Ha at 5.95 ppm is also a doublet of doublets, with coupling constants 3Jac= 17.4 Hz and 3Jab = 10.5 Hz. We know it is not a quartet because the intensity of the lines is the same for each like a doublet, whereas for a quartet the ratio of the lines is 1:3:3:1 where the outside lines would be shorter.
The signal for Hb at 5.64 ppm is split into a doublet by Ha, a cis coupling with 3Jab = 10.4 Hz. Each of the resulting sub-peaks is split again by Hc, with the same geminal coupling constant 2Jbc = 1.5 Hz that we saw previously when we looked at the Hc signal. The overall result is again a doublet of doublets, this time with the two `sub-doublets` spaced slightly closer due to the smaller coupling constant for the cis interaction. Here is a blow-up of the actual Hb signal:
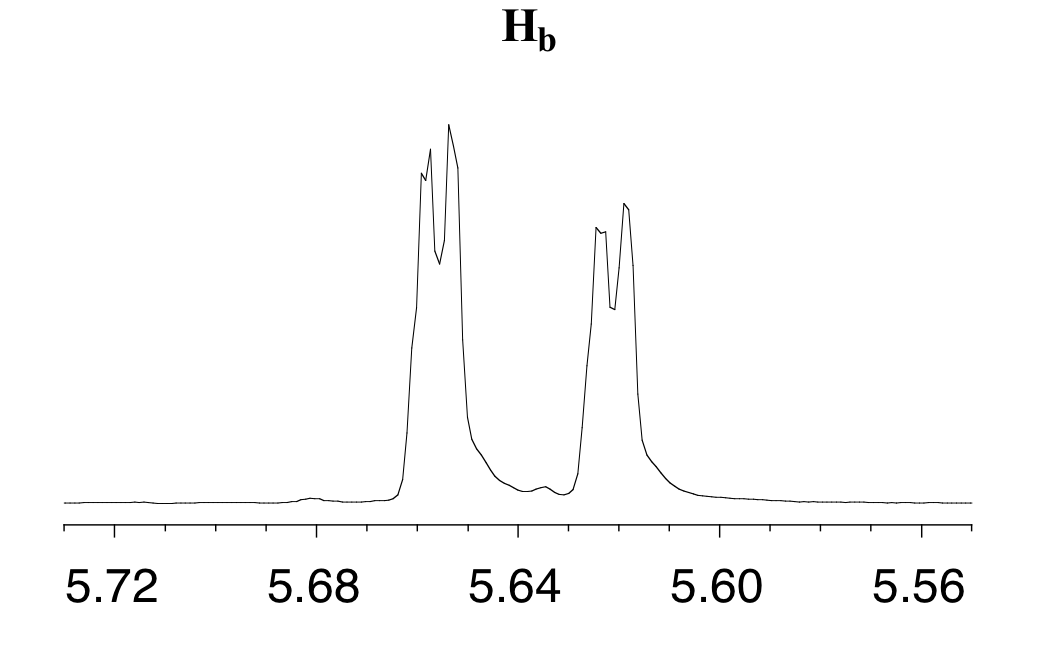
You may have noticed in the splitting diagram, the larger of coupling constant came first followed by the smaller or finer coupling constant.While it does not matter which coupling constant you start with, it is usually easier to construct the splitting diagram for analysis of complex coupling patterns if you begin with the larger coupling constant. However, the end result will be the same regardless of how you begin.
Construct a splitting diagram for the Hb signal in the 1H-NMR spectrum of methyl acrylate. Show the chemical shift value for each sub-peak, expressed in Hz (assume that the resonance frequency of TMS is exactly 300 MHz).
Solution
The coupling tree for Hb is:
The complex coupling pattern would be a doublet of doublets (dd).
In many cases, it is difficult to fully analyze a complex splitting pattern. Aromatic ring protons quite commonly have overlapping signals and multiplet distortions. Sometimes you cannot distinguish between individual signals, and one or more messy multiplets often appear in the aromatic region. In the spectrum of toluene, for example, if we consider only 3-bond coupling we would expect the signal for Ha to be a doublet, Hb a triplet, and Hc a triplet.
In practice, however, all three aromatic proton groups have very similar chemical shifts and their signals overlap substantially, making such detailed analysis difficult. In this case, we would refer to the aromatic part of the spectrum (~7.0 ppm) as a multiplet.

When we start trying to analyze complex splitting patterns in larger molecules, we gain an appreciation for why scientists are willing to pay large sums of money (hundreds of thousands of dollars) for higher-field NMR instruments. Quite simply, the stronger our magnet is, the more resolution we get in our spectrum. In a 100 MHz instrument (with a magnet of approximately 2.4 Tesla field strength), the 12 ppm frequency 'window' in which we can observe proton signals is 1200 Hz wide. In a 500 MHz (~12 Tesla) instrument, however, the window is 6000 Hz - five times wider. In this sense, NMR instruments are like digital cameras and HDTVs: better resolution means more information and clearer pictures (and higher price tags!).
It is much easier to rationalize the observed 1H NMR spectrum of a known compound than it is to determine the structure of an unknown compound from its 1H NMR spectrum. However, rationalizations can be a useful learning technique as you try to improve your proficiency in spectral interpretation. Remember that when a chemist tries to interpret the 1H NMR spectrum of an unknown compound, he or she usually has additional information available to make the task easier. For example, the chemist will almost certainly have an infrared spectrum of the compound and possibly a mass spectrum too. Details of how the compound was synthesized may be available, together with some indication of its chemical properties, its physical properties, or both.
Exercises
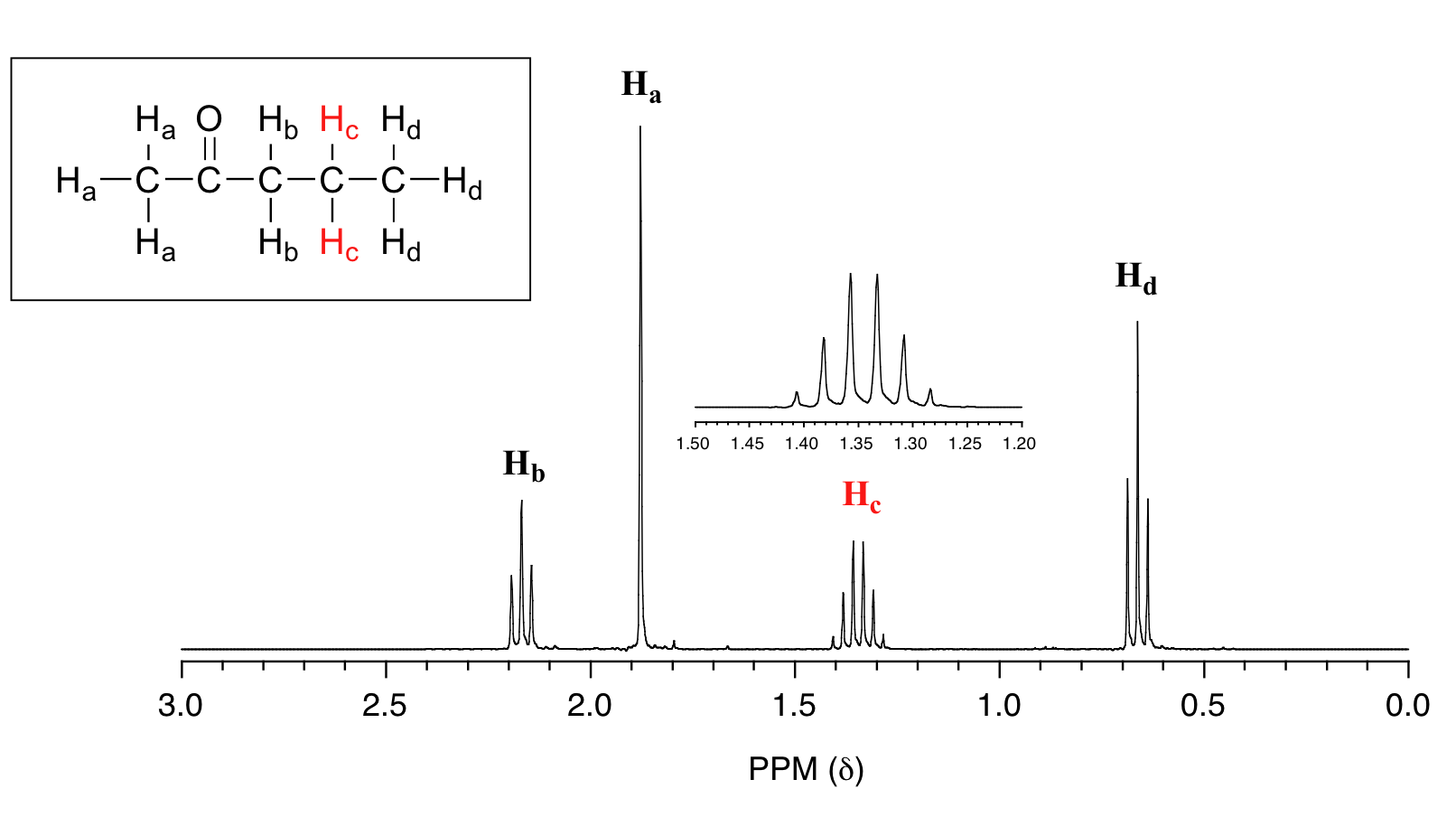
The molecule below is 2-pentanone, what would the splitting pattern be for the two hydrogens explicitly indicated (below) in an 1H NMR spectrum.
- Answer
-
The indicated hydrogens (Hc below) in the spectrum of 2-pentanone appears as a sextet. They would be split by the five combined protons (Hb and Hd below). Since the intervening bonds are the same, Jbc and Jcd would be very similar and the n+1 rule can be followed. Technically, this 'sextet' could be considered to be a 'triplet of quartets' with overlapping sub-peaks.
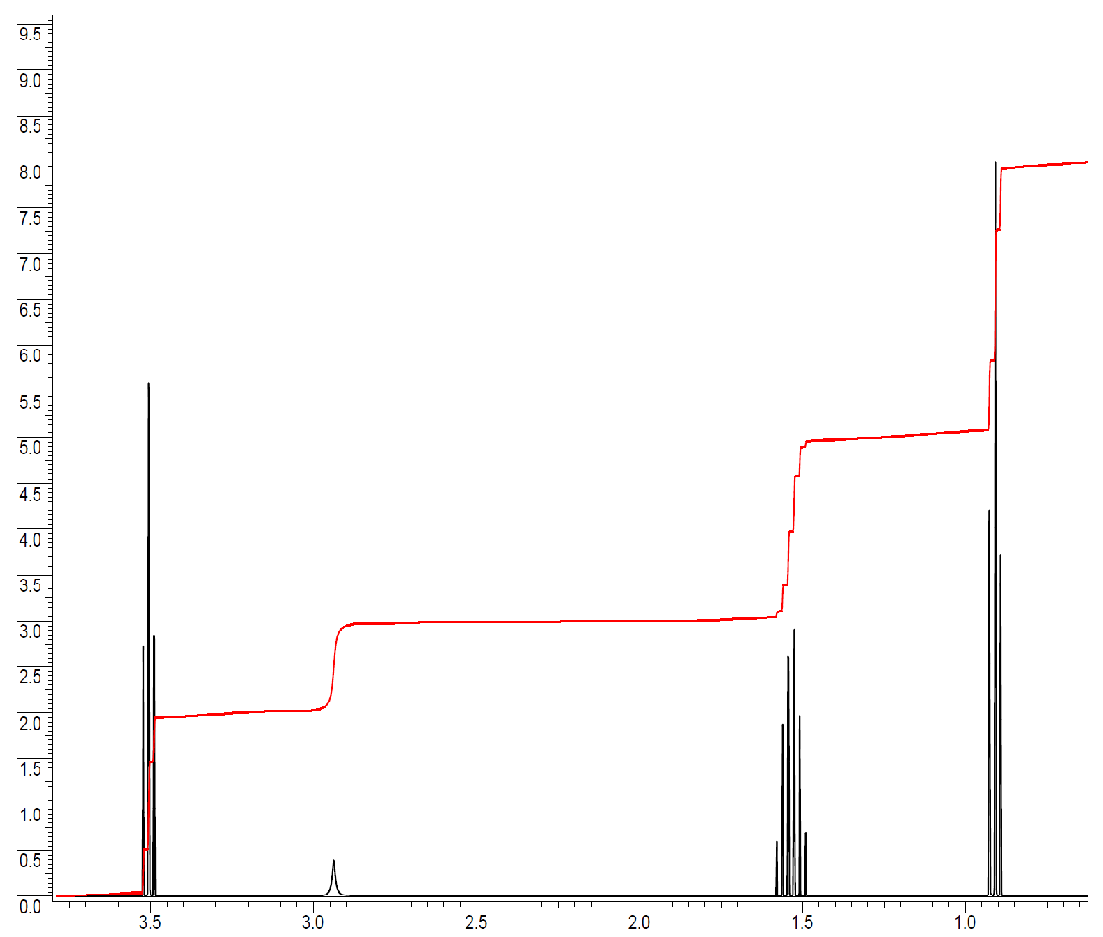
The following spectrum is for C3H8O. Determine the structure. The ratio of integrals from left to right is 2:1:2:3.
- Answer
-
The total number of hydrogens is 2+1+2+3 = 8. All the hydrogens are accounted for.
Typically, integrations of 3 or multiples of 3 are methyl groups. The peak at 0.9 ppm is a methyl group with two neighbors, since the peak is split into a triplet. n +1 = 3, n = 2, n = number of neighbors. The first fragment is a -CH2-CH3.
The peak just above 1.5 ppm integrates to 2 protons and is a sextet (split into 6 peaks). The integration of 2 indicates this is a methylene group (-CH2-) and the spin-spin splitting pattern tells us that there are 5 neighbors. A methylene makes two more bonds, so one side must be the methyl group and the other side must be another methylene in order to have 5 total neighbors. The next fragment is -CH2-CH2-CH3.
The peak just below 3.0 ppm is a broad singlet. Broad singlets typically come from exchangeable protons like an -OH or -NH-. In our molecular formula we have no N, but we do have an O, so this peak must be due to the -OH.
The final peak at 3.5 ppm integrates to 2 and is a triplet. The integration indicates it is another methylene group and since it is a triplet, it must have two neighbors. So far, the fragment would be -CH2-CH2-. It is also very far downfield for a proton just bonded to other carbons with hydrogens, which would typically be around 1.2-1.7 ppm (see the table in Section 5.5). This means it must be attached to an electron withdrawing group. The electron withdrawing group in this molecule is the -OH. Our final fragment is -CH2-CH2-OH.
If we look at all of our fragments, we can see that it is too many carbons and hydrogens if they all represented a separate piece to include. However, spin-spin splitting is the information from neighboring hydrogens. This means that the fragments overlap each other. Let's take the first two fragments (-CH2-CH3 and -CH2-CH2-CH3). The integration information indicates that there is only one peak that integrates to 3, so there is only one methyl group, not two. This means one of the methyl groups is a duplicate and there is actually only one methyl group. In the second fragment, the splitting pattern indicated that there must be a neighboring methyl group, but the information is actually about the central methylene in that fragment. Considering this, the molecule must be HO-CH2-CH2-CH3. It has four different types of hydrogens, with the correct ratios as well as splitting pattern.
For the molecule below, what would the coupling pattern be in a 1H NMR spectrum?
- Answer
-
Hb would be a doublet of doublet of triplets. Hc splits Hb by trans coupling into a doublet. Ha splits Hb by cis coupling into a doublet. The two Hd protons split Hb into a triplet. All of the J coupling constants are different, so you cannot use the n+1 rule.
Unknown compound D (C15H14O) has the following spectral properties.
Infrared spectrum:
3010 cm−1 (medium)
1715 cm−1 (strong)
1610 cm−1 (strong)
1500 cm−1 (strong)
1H NMR spectrum:
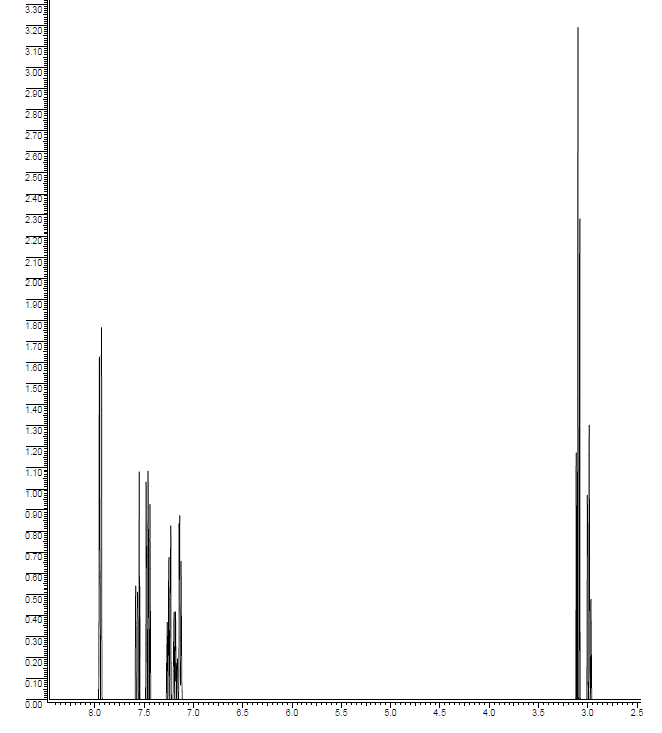
| δ (ppm) | Relative Area | Multiplicity |
|---|---|---|
| 3.00 | 2 | triplet |
| 3.07 | 2 | triplet |
| 7.1-7.9 | 10 | Multiplets |
- Answer
-
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)

