17.3: Thiamine Diphosphate (Vitamin B1)
- Page ID
- 106401
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Thiamine diphosphate (\(ThDP\), sometimes also abbreviated \(TPP\) or \(ThPP\)) is a coenzyme which, like PLP, acts as an electron sink to stabilize key carbanion intermediates. The most important part of the \(ThDP\) molecule from a catalytic standpoint is its thiazole ring.
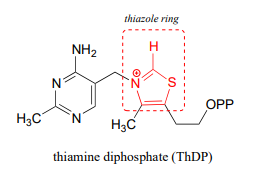
The proton on the carbon between nitrogen and sulfur on the thiazole ring is weakly acidic, with a \(pK_a\) of about 18.
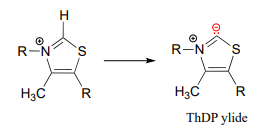
The reason for its acidity lies partly in the ability of the neighboring sulfur atom to accept, in its open \(d\)-orbitals, some of the excess electron density of the conjugate base. Another reason is that the positive charge on the nitrogen helps to stabilize the negative charge on the conjugate base. The deprotonated thiazole is called an ylide, which is a general term for a species with adjacent positively and negatively charged atoms.
The negatively charged carbon on the ylide form of \(ThDP\) is nucleophilic, and as we shall soon see, the first step of most \(TPP\)-dependent reactions is nucleophilic attack of the ylide carbon on a carbonyl group of the reaction substrate.
\(ThDP\) plays a key role in a variety of reaction types, but the common theme in all \(ThDP\)-dependent reactions is cleavage of a bond adjacent to the carbonyl carbon of a ketone or aldehyde.
Thiamine diphosphate assists in breaking bonds next to a ketone or aldehyde:
Consider this hypothetical decarboxylation step:
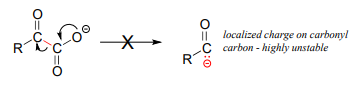
Hopefully, you can quickly recognize that this is not a chemically reasonable step, because the intermediate species which results from decarboxylation has a negative charge localized on the ketone carbon - a very unstable, unlikely intermediate indeed.
(Recall from section 13.1 that decarboxylation steps usually result in intermediates in which the negative formal charge is delocalized to an oxygen or nitrogen - in other words, enolates or enamines.)
Now consider, however, a reaction going on in your cells right now, catalyzed by the enzyme pyruvate decarboxylase (EC 4.1.1.1):
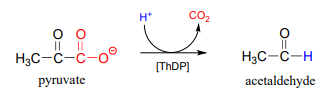
Somehow, the enzyme manages to accomplish this 'impossible' decarboxylation. How does this happen? Here is where the thiamine diphosphate coenzyme comes in.
A \(ThDP\)-dependent decarboxylation reaction (pyruvate decarboxylase):
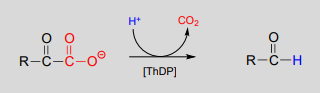
Mechanism:
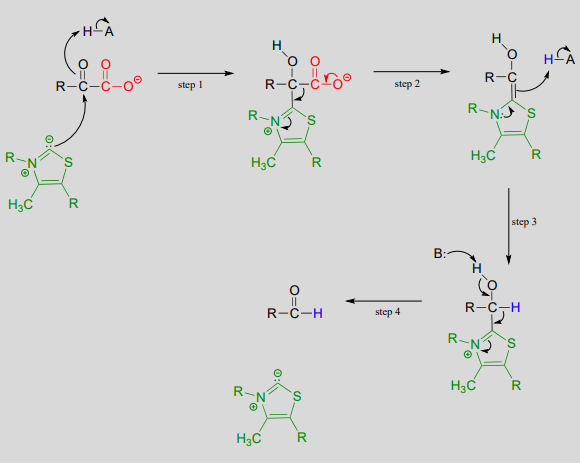
Upon binding to the enzyme's active site, \(ThDP\) quickly loses a proton. The nucleophilic ylide carbon then adds to the carbonyl carbon of pyruvate.
Look carefully at the intermediate that results from step 1 in the mechanism above. The thiazole ring of \(ThDP\), once it has added to the carbonyl of pyruvate, provides an 'electron sink' to absorb the electrons from decarboxylation (step 2). Note which bond is breaking in step 2 - as was mentioned earlier, the common function of \(ThDP\) is to make possible the cleavage of a bond to a carbonyl carbon.
In step 3, the electrons from decarboxylation flow back to abstract a proton from an acidic group in the active site. All that remains is for the product to break free of thiamine in step 4.
Thiamine can also assist in decarboxylation-addition reactions:
\(ThDP\)-dependent decarboxylation-addition:
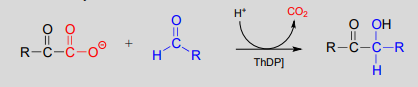
Mechanism:
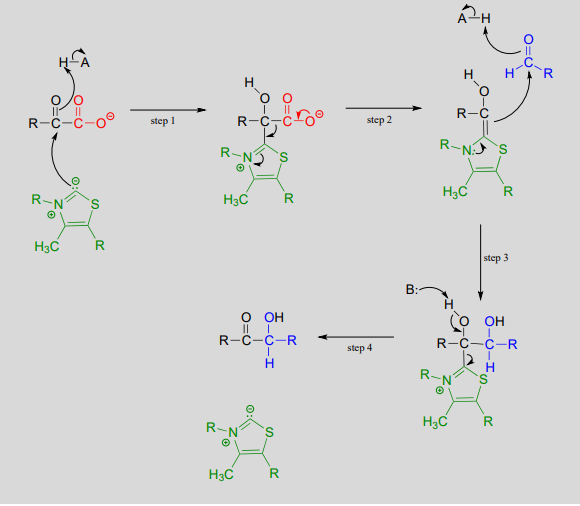
Here, the electron-rich intermediate formed from the decarboxylation step (step 2) simply goes on to act as a nucleophile rather than as a base, adding to the carbonyl group of an aldehyde or ketone (step 3). As before, the product breaks free of \(ThDP\) in step 4.
An example is the first step in the biosynthetic pathway for isoprenoid compounds in bacteria:
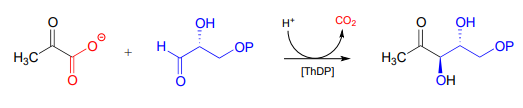
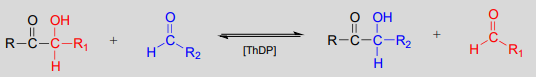
Transketolase, a \(ThDP\)-dependent enzyme in the pentose phosphate pathway of sugar metabolism, catalyzes a carbon-carbon bond break step, followed by a carbon-carbon bond forming step. The substrates and products are at similar energy levels, so the reaction is completely reversible.
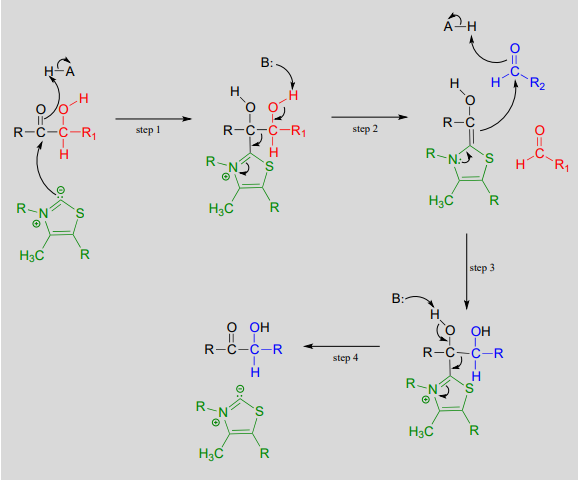
Transketolase reaction:
Mechanism:
Below is an actual example of a transketolase-catalyzed transformation from the pentose phosphate pathway (shown in Fischer projections, as is common for sugar structures).
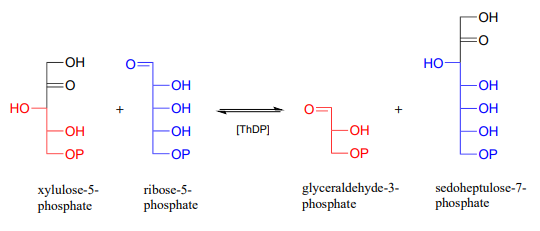
As was mentioned above, the transketolase reaction is highly reversible. Do you think the same can be said for the decarboxylation and decarboxylation-addition reactions we saw in this section? Why or why not?
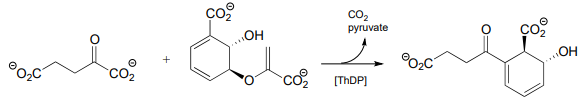
(Challenging!) Propose a mechanism for the reaction below.
- Hint
-
This is a \(ThDP\)-facilitated decarboxylation/ Michael addition, followed by \(E1cb\) elimination of pyruvate. A Michael addition is the name for a conjugate addition with a carbon nucleophile. (J. Mol. Biol. 2010, 401, 253).
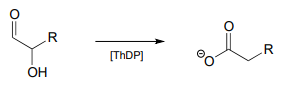
Propose a mechanism for the reaction below.
- Hint
-
The mechanism can be described as a \(ThDP\)-facilitated dehydration step, followed by a tautomerization step, followed by a hydrolytic expulsion of \(ThDP\) (a different kind of \(ThDP\) expulsion from what we have seen so far!)


