16.2: Overview of Single-Electron Reactions and Free Radicals
- Page ID
- 106393
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Beginning with acid-base reactions in chapter x and continuing though the chapters on nucleophilic substitution, carbonyl addition, acyl substitution, a-carbon chemistry, and electrophilic reactions , we have been studying reaction mechanisms in which both electrons in a covalent bond or lone pair move in the same direction. In this chapter, we learn about reactions in which the key steps involve the movement of single electrons. Single electron movement is depicted by single-barbed 'fish-hook' arrows (as opposed to the familiar double-barbed arrows that we have been using throughout the book to show two-electron movement).

Single-electron mechanisms involve the formation and subsequent reaction of free radical species, highly unstable intermediates that contain an unpaired electron. Free radicals are often formed from homolytic cleavage, an event in which the two electrons in a breaking covalent bond move in opposite directions. The bond in molecular chlorine, for example, is subject to homolytic cleavage when chlorine is subjected to heat or light. The result is two chlorine radicals. Note that each radical has a formal charge of zero.
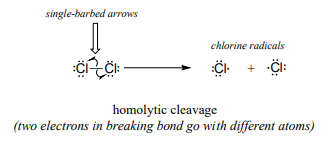
In contrast, essentially all of the reactions we have studied up to now involve bond-breaking events in which both electrons move in the same direction: this is called heterolytic cleavage.

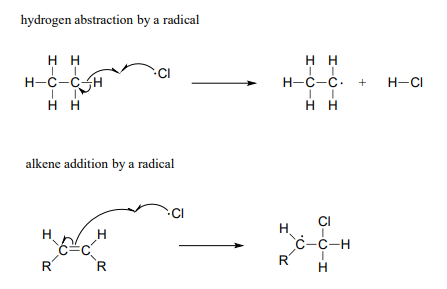
Two other homolytic cleavage reactions that we will see in this chapter can be described as 'radical hydrogen atom abstraction' and 'radical alkene addition':
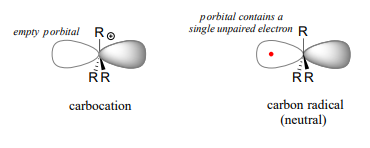
Single-electron reaction mechanisms involve the formation of radical species, and in organic reactions these are often carbon radicals. A carbon radical is \(sp^2\) hybridized, with three s bonds arranged in trigonal planar geometry and the single unpaired electron occupying an unhybridized p orbital. Contrast this picture with a carbocation reactive intermediate, which is also \(sp^2\) hybridized with trigonal planar geometry but with an empty p orbital.
When we studied electrophilic reactions in chapter 14, a major concern when evaluating possible mechanisms was the stability of any carbocation intermediate(s). Likewise, the stability of proposed radical intermediates is of great importance when evaluating the likelihood of possible single-electron mechanisms. Fortunately, the trend in the stability of carbon radicals parallels that of carbocations (section 8.5): tertiary radicals, for example, are more stable than secondary radicals, followed by primary and methyl radicals. This should make intuitive sense, because radicals, like carbocations, are electron deficient, and thus are stabilized by the electron-donating effects of nearby alkyl groups.

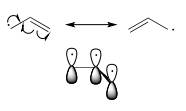
Benzylic and allylic radicals are more stable than alkyl radicals due to resonance effects - an unpaired electron (just like a positive or negative charge) can be delocalized over a system of conjugated π bonds. An allylic radical, for example, can be pictured as a system of three parallel p orbitals sharing three electrons.
The drawing below shows how a benzylic radical is delocalized to three additional carbons around the aromatic ring:
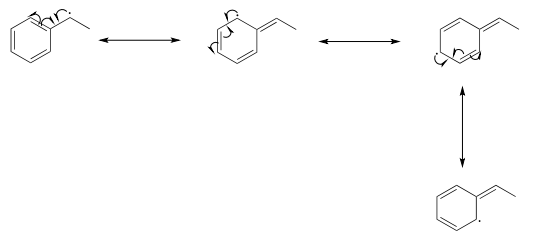
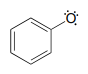
Just as phenolate ions are less reactive (less basic) than alkoxide ions, phenolic radicals are less reactive than alkoxide radicals. Draw one resonance contributor of a phenolic radical showing how the radical electron is delocalized to a ring carbon. Include electron-movement arrows.
While radical species are almost always very reactive and short-lived, in some extreme cases they can be unreactive. One example of an inert organic radical structure is shown below.
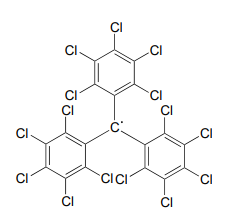
The already extensive benzylic resonance stabilization is further enhanced by the fact that the large electron clouds on the chlorine atoms shield the radical center from external reagents. The radical is, in some sense, inside a protective 'cage'.
Draw a resonance contributor of the structure above in which the unpaired electron is formally located on a chlorine atom (include electron movement arrows)


