16.1: Prelude to Radical Reactions
- Page ID
- 106392
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
Introduction
Imagine that you are an 18th century British sailor about set out with Commodore George Anson to raid Spanish shipping fleets in the Pacific. You know full well that you are signing up for a long and arduous ordeal, with months of constant seasickness, bad food, cramped, unsanitary conditions, and brutal warfare. You are mentally ready for these hardships, but what you are not prepared for is to watch your own body rot away – to literally fall apart.
Below is a description of the suffering endured by many sailors of the time:
Some lost their very substance and their legs became swollen and puffed up while the sinews contracted and turned coal-black and, in some cases, all blotched with drops of purplish blood. Then the disease would creep up to the hips, thighs and shoulders, arms and neck. And all the sick had their mouths so tainted and their gums so decayed that the flesh peeled off down to the roots of their teeth, which almost all fell out. . .
There were devastating neurological as well as physiological effects. Scurvy had the ability to inhibit a person's normal restraints on emotion: they became intensely homesick and nostalgic, wept at the slightest disappointment, and screamed in agony upon smelling the scent of flower blossoms drifting across the water from a nearby shore.
The disease afflicting the sailors was scurvy, which we now know is caused by a deficiency of vitamin C in the diet. European sea voyagers in the 18th century and earlier subsisted mainly on a diet of salted meat, hard biscuits, pea soup, oatmeal, and beer. After the first couple of weeks at sea, fresh fruits and vegetables - and the nutrients they contained – were all consumed or spoiled. The salted meat and hardtack diet provided salt and calories, but little else of nutritional value.
Although it is rare now, scurvy has plagued sailors for centuries, with records of its occurrence on ships going back as far as the 15th century voyages of Magellan and Vasco de Gama, both of whom lost up to three quarters of their crew to the disease on long ocean crossings. Various cultures made the connection between scurvy and diet, and learned effective preventative measures: sailors with the 16th century French explorer Jacques Cartier, for example, were cured of their scurvy upon arriving in Canada and taking the advice of native people to eat the leaves and bark of pine trees. These were lessons, unfortunately, that often had to be relearned time and again, as the knowledge gained by one culture was not effectively recorded and passed along to others.
Vitamin C, or ascorbic acid as it is known to chemists, plays an essential helping role in a variety of essential biochemical reactions. Most living things are able to synthesize ascorbic acid – the exceptions include humans and other higher primates, several species of bats, and some rodents such as guinea pigs and capybaras. Humans lack the last enzyme in the ascorbic acid biosynthetic pathway, L-gulonolactone oxidase. (EC 1.1.3.8) (You were invited to propose the mechanism for this redox reaction in problem 15.10).
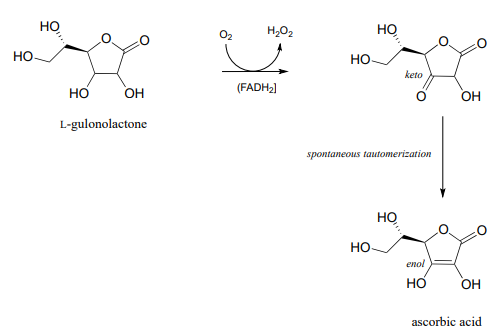
Because we cannot make our own ascorbic acid, we need to get it in our diet. It is abundant in many plant-based foods, citrus fruits in particular. The traditional diet of the Inuit people of the arctic region contains virtually no plant products, but vitamin C is obtained from foods such as kelp, caribou livers, and whale skin. For a time in the 18th century, the observation that citrus fruits quickly cured scurvy led to the practice of including in a ship's stores a paste prepared from boiled lemon juice. Unfortunately, ascorbic acid did not survive the boiling process, rendering the paste ineffective against scurvy. Captain James Cook, the legendary explorer and the first European to make it to the east coast of Australia and the Hawaiian islands, brought along sourkraut (fermented cabbage), a somewhat more effective vitamin C supplement. According to his own account, Cook's sailors at first refused to eat the pungent preparation, so the captain engaged in a little psychological trickery: he declared that it would only be served to officers. The enlisted sailors quickly took offense, and demanded their own sourkraut ration. Later, the British navy famously adopted the practice of adding lemon or lime juice to their ships' rum rations, leading to the birth of the slang term 'Limey' used by European and American sailors to refer to their British counterparts.
The biochemical role of ascorbic acid is to facilitate the transfer of single electrons in a variety of redox reactions - note here the emphasis on single electrons, as opposed to the redox reactions we studied in chapter 15 in which electrons were transferred in pairs. The subject of this chapter is single-electron chemistry, and the free radical intermediates that are involved in single electron reaction steps.
Later in this chapter we will learn the chemical details of why ascorbic acid deficiency causes scurvy, how the act of breathing makes you get old, how polystyrene packing foam is made, and other interesting applications of single-electron chemistry. But first we need to cover some basics ideas about single electron chemical steps, and the free radical intermediates that result from them.


