11.10: Nucleophilic Acyl Substitution Reactions in the Laboratory
- Page ID
- 106596
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)All of the biological nucleophilic acyl substitution reactions we have seen so far have counterparts in laboratory organic synthesis. Mechanistically, one of the biggest differences between the biological and the lab versions is that the lab reactions usually are run with a strong acid or base as a catalyst, whereas biological reactions are of course taking place at physiological \(pH.\) When proposing mechanisms, then, care must be taken to draw intermediates in their reasonable protonation states: for example, a hydronium ion (\(H_3O^+\)) intermediate is reasonable to propose in an acidic reaction, but a hydroxide (\(OH^-\)) intermediate is not.
Ester reactions - bananas, soap and biodiesel
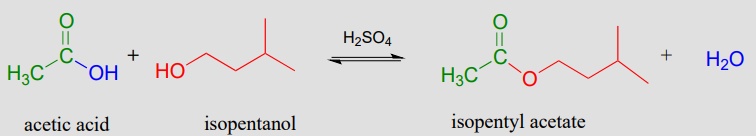
Acid-catalyzed synthesis of flavor compounds such as isopentyl acetate (an ester with the flavor of banana) is simple to carry out in the lab. In this esterification reaction, acetic acid is combined with isopentyl alcohol along with a catalytic amount of sulfuric acid.
Acid-catalyzed esterification (laboratory reaction):
Mechanism:
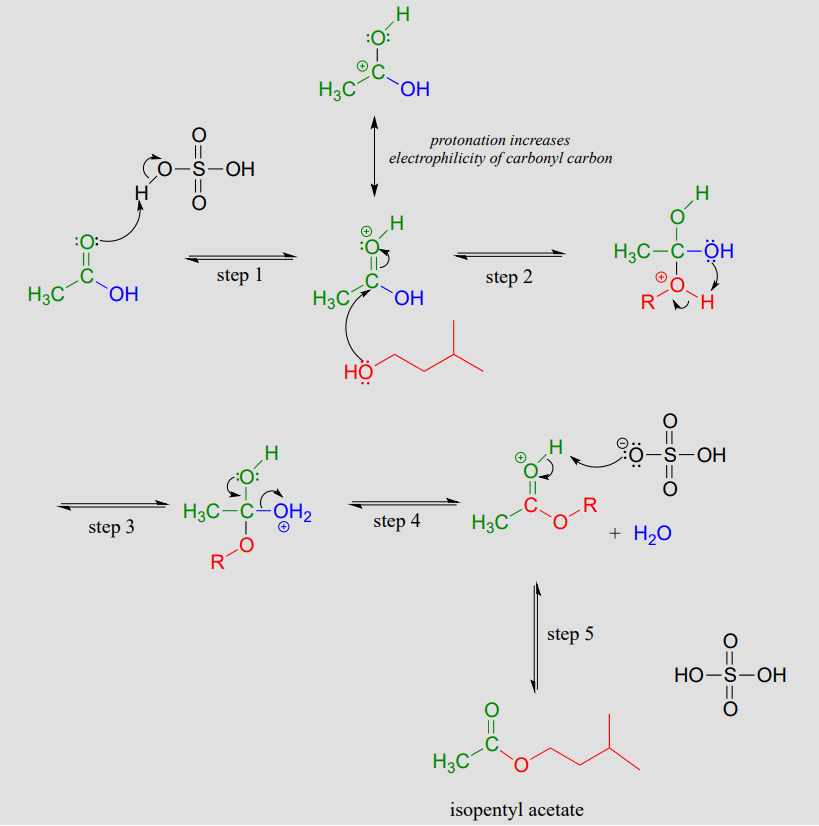
The carbonyl oxygen of acetic acid is first protonated (step 1), which draws electron density away from the carbon and increases its electrophilicity. In step 2, the alcohol nucleophile attacks: notice that under acidic conditions, the nucleophile is not deprotonated simultaneously as it attacks (as we would show in a biochemical mechanism), and the tetrahedral intermediate is a cation rather than an anion. In step 3, a proton is transferred from one oxygen atom to another, creating a good leaving group (water) which is expelled in step 4. Finally (step 5), the carbonyl oxygen on the ester is deprotonated, regenerating the catalytic acid.
This reaction is highly reversible, because carboxylic acids are approximately as reactive as esters. In order to obtain good yields of the ester, an excess of acetic acid can be used, which by Le Chatelier's principle (see your General Chemistry textbook for a review) shifts the equilibrium toward the ester product.
Saponification is a common term for base-induced hydrolysis of an ester. For example, methyl benzoate will hydrolize to benzoate and methanol when added to water with a catalytic amount of sodium hydroxide.
Mechanism of base-catalyzed ester hydrolysis (saponification):
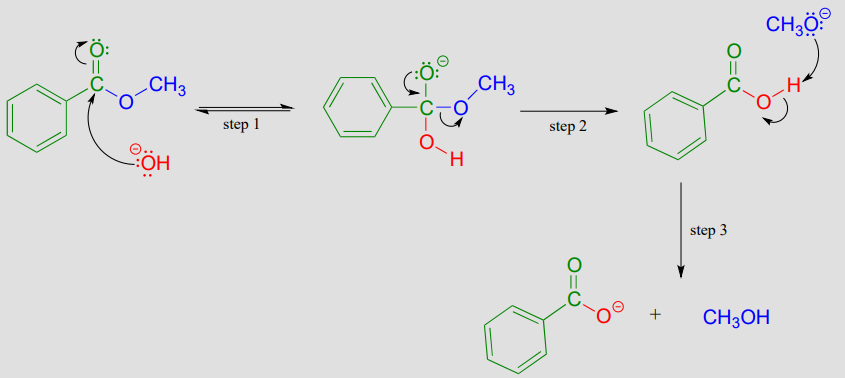
Addition of the base provides hydroxide ion to act as a nucleophile (hydroxide is of course a better nucleophile than water) in step 1. The tetrahedral intermediate (anionic in this case, because the reaction conditions are basic) then collapses in step 2, and the alkoxide (\(CH_3O^-\)) leaves. We are not used to seeing alkoxides or hydroxides as leaving groups in biochemical reactions, because they are strong bases - but in a basic solution, this is a reasonable chemical step. Step 3 is simply an acid-base reaction between the carboxylic acid and the alkoxide. Note that this is referred to as base-induced rather than base-catalyzed because hydroxide is not regenerated, and thus a full molar equivalent of base must be used.
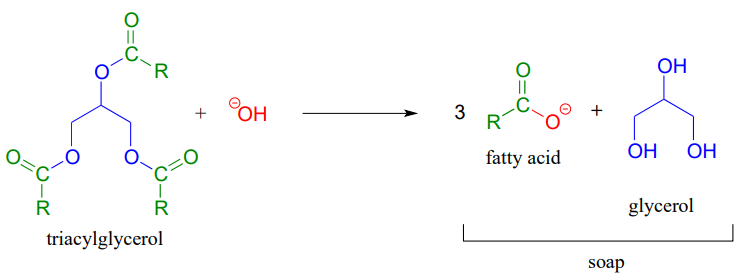
The saponification process derives its name from the ancient craft of soap-making, in which the ester groups of triacylglycerols in animal fats are hydrolized under basic conditions to glycerol and fatty acyl anions (see section 2.5A for a reminder of how fatty acyl anions work as soap).
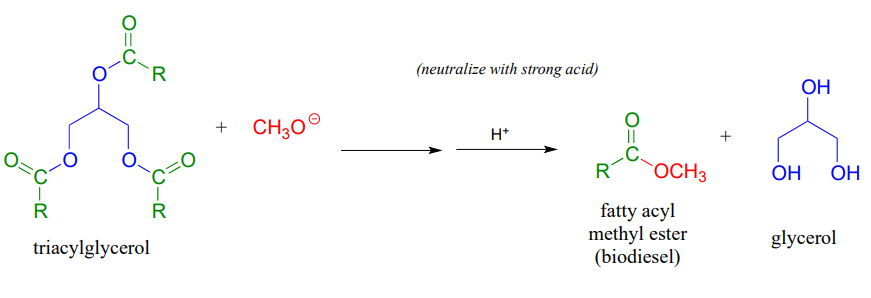
We learned earlier about transesterification reactions in the context of the chemical mechanism of aspirin. Transesterification also plays a key role in a technology that is already an important component in the overall effort to develop environmentally friendly, renewable energy sources: biodeisel. You may have heard stories about people running their cars on biodeisel from used french fry oil. To make biodeisel, triacylglycerols in fats and oils can be transesterified with methanol or ethanol under basic conditions. The fatty acyl methyl and ethyl ester products are viable motor fuels.
Draw structures of the carboxylic acid and alcohol starting materials that could be used to synthesize the fragrant fruit esters shown in section 11.1.
What would happen if you tried to synthesize isopentyl acetate (banana oil) with basic rather than acidic conditions? Would this work?
Consider the reverse direction of the acid-catalyzed esterification reaction. What would you call this reaction in organic chemistry terms?
An alternative way to synthesize esters is to start with a carboxylate and an alkyl halide. Draw a mechanism for such a synthesis of methyl benzoate - what type of reaction mechanism is this?
Acid chlorides and acid anhydrides
In the cell, acyl phosphates and thioesters are the most reactive of the carboxylic acid derivatives. In the organic synthesis lab, their counterparts are acid chlorides and acid anhydrides, respectively. Of the two, acid chlorides are the more reactive, because the chloride ion is a weaker base and better leaving group than the carboxylate ion (the \(pK_a\) of \(HCl\) is -7, while that of carboxylic acids is about 4.5: remember, a stronger conjugate acid means a weaker conjugate base).
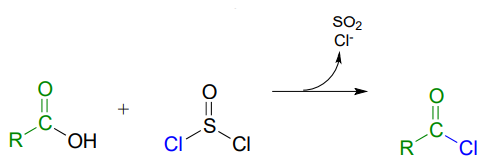
Acid chlorides can be prepared from carboxylic acids using \(SOCl_2\):
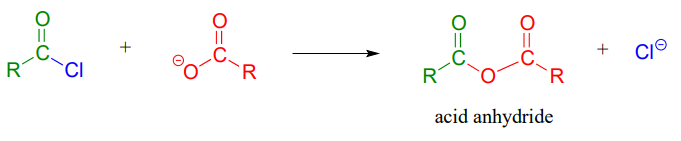
Acid anhydrides can be prepared from carboxylic acids and an acid chloride under basic conditions:
Acetic anhydride is often used to prepare acetate esters and amides from alcohols and amines, respectively. The synthesis of aspirin and acetaminophen are examples:
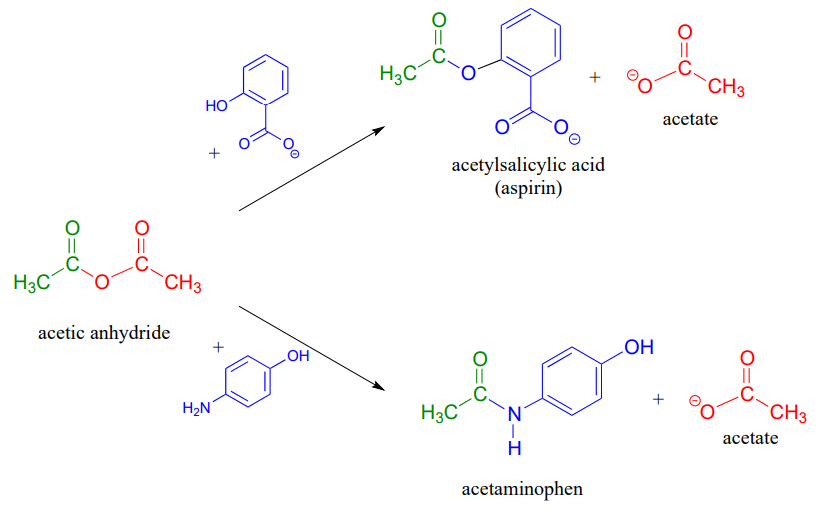
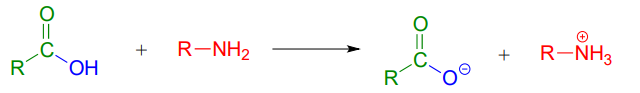
A carboxylic acid cannot be directly converted into an amide because the amine nucleophile would simply act as a base and deprotonate the carboxylic acid:
Instead, the carboxylic acid is first converted to an acid chloride (in other words, the carboxylic acid is activated), then the acid chloride is combined with an amine to make the amide.
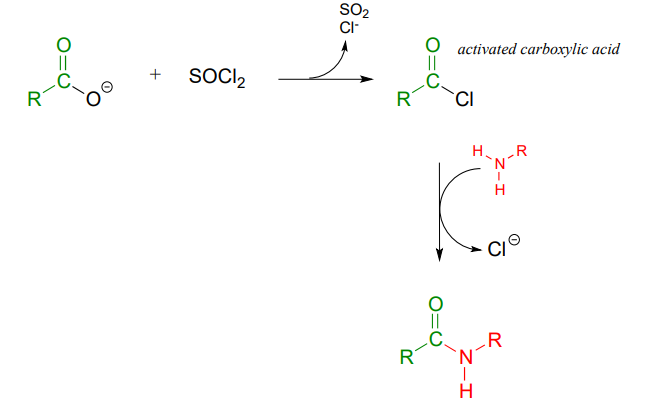
This sequence of reactions is a direct parallel to the biochemical glutamine and asparagine synthase reactions we saw earlier in the chapter (section 11.5), except that the activated form of carboxylic acid is an acid chloride instead of an acyl phosphate or acyl-AMP.
For the preparation of the amide below, show a starting carboxylate and amine and the intermediate acid chloride species.
Polyesters and polyamides
If you have ever had the misfortune of undergoing surgery or having to be stitched up after a bad cut, it is likely that you benefited from our increasing understanding of polymers and carboxylic ester chemistry. Polyglycolic acid is a material commonly used to make dissolving sutures. It is a polyester - a polymer linked together by ester groups - and is formed from successive acyl substitution reactions between the alcohol group on one end of a glycolic acid monomer and the carboxylic acid group on a second:
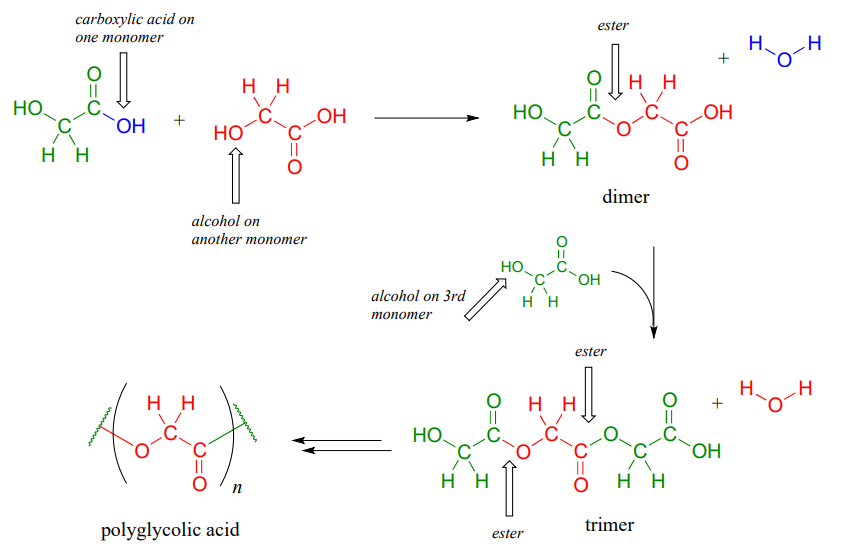
The resulting polymer - in which each strand is generally several hundred to a few thousand monomers long - is strong, flexible, and not irritating to body tissues. It is not, however, permanent: the ester groups are reactive to gradual, spontaneous hydrolysis at physiological \(pH\), which means that the threads will dissolve naturally over several weeks, eliminating the need for them to be cut out by a doctor.
Dacron, a polyester used in clothing fiber, is made of alternating dimethyl terephthalate and ethylene glycol monomers.
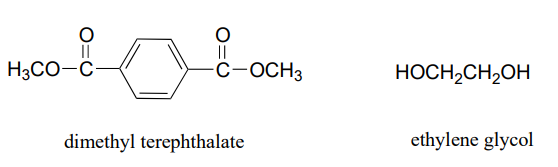
- Draw the structure of a Dacron tetramer (in other words, four monomers linked together).
- Water is a side product of glycolic acid polymerization. What is the equivalent side product in Dacron production?
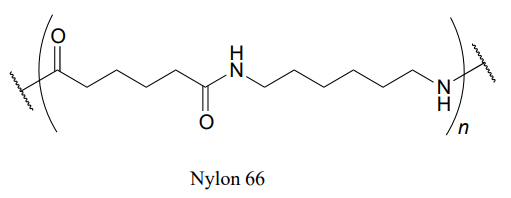
Nylon 6,6 is a widely used polyamide composed of alternating monomers. Nylon 6,6 has the structure shown below -the region within the parentheses is the repeating unit, with 'n' indicating a large number of repeats. Identify the two monomeric compounds used to make the polymer.
The Gabriel synthesis of primary amines
The Gabriel synthesis, named after the 19th-century German chemist Siegmund Gabriel, is a useful way to convert alkyl halides to amines and another example of \(S_N2\) and acyl substitution steps in the laboratory. The nitrogen in the newly introduced amine group comes from phthalimide. In the first step of the reaction, phthalimide is deprotonated by hydroxide, then in step 2 it acts as a nucleophile to displace a halide in an \(S_N2\) reaction (phthalimide is not a very powerful nucleophile, so this reaction works only with unhindered primary or methyl halides).
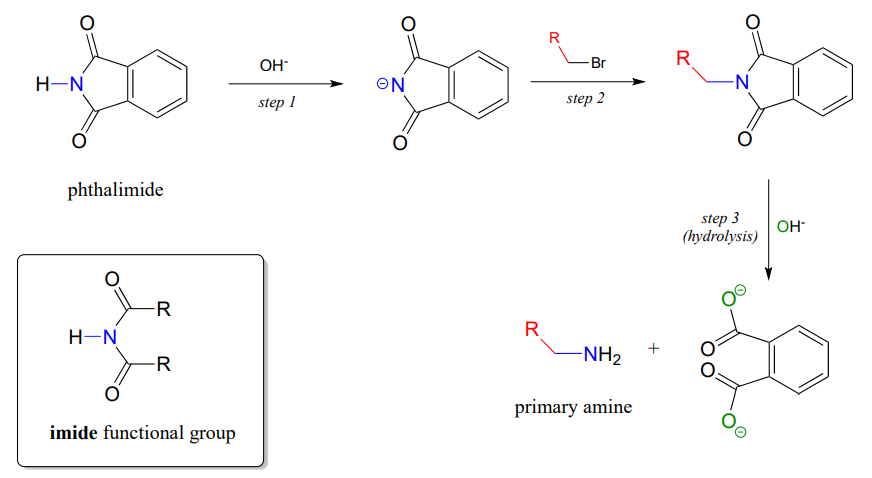
Step 3 is simply a pair of hydrolytic acyl substitution steps to release the primary amine, with an aromatic dicarboxylate by-product.
Phthalimide contains an 'imide' functional group, and has a \(pK_a\) of approximately 10. What makes the imide group so much more acidic than an amide, which has a \(pK_a\) of approximately 17?
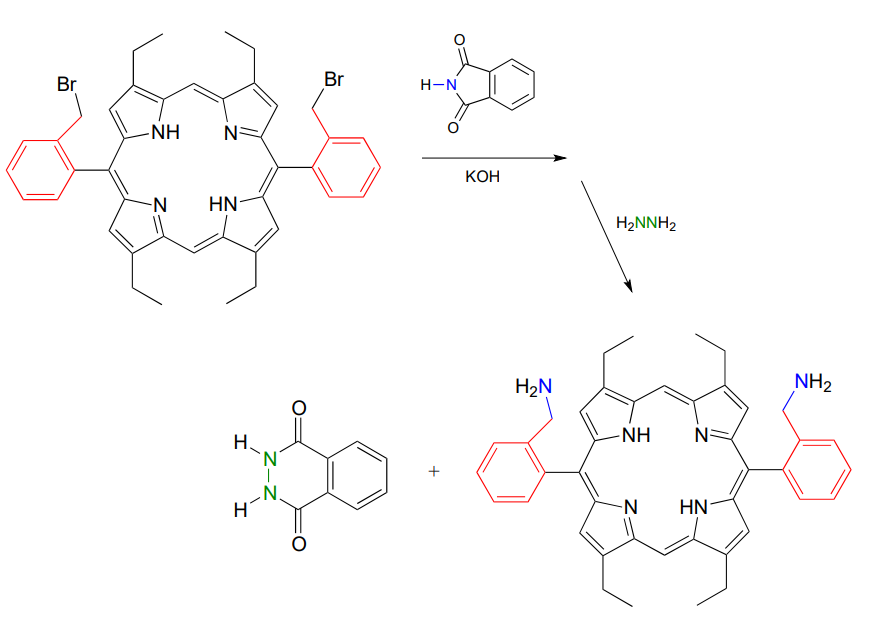
As an alternative procedure, release of the amine in step 3 can be carried out with hydrazine (\(H_2NNH_2\)) instead of hydroxide. Again, this occurs through two nucleophilic acyl substitution reactions.
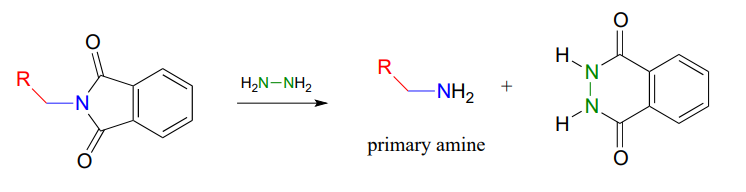
In 2000, chemists at MIT synthesizing a porphyrin-containing molecule introduced two amine groups using the Gabriel synthesis with hydrazine. Porphyrins, which include the 'heme' in our red blood cells, are an important family of biomolecules with a variety of biochemical function (J. Org. Chem. 2000, 65, 5298).