11.11: A Look Ahead - Acyl Substitution Reactions with a Carbanion or Hydride Ion Nucleophile
- Page ID
- 106597
Although we have seen many different types of nucleophilic acyl substitutions in this chapter, we have not yet encountered a reaction in which the incoming nucleophile is a carbanion or a hydride. Recall that in the previous chapter on aldehydes and ketones, we also postponed discussion of nucleophilic carbonyl addition reactions in which a carbanion or a hydride is the nucleophile. The reason for putting off these discussions is that these topics are both important and diverse enough to warrant their own dedicated chapters.
In the next chapter, we will see many carbonyl addition and acyl substitution reactions where the nucleophilic species is a resonance-stabilized carbanion such as an enolate (section 7.6). Then in chapter 14, we will encounter nucleophilic addition and acyl substitution reactions in which a hydride ion (\(H^-\)) essentially plays the part of a nucleophile. In these chapters we will see how nucleophilic carbanion and hydride species are generated in a biochemical context. For now, see if you can predict the result of the following biochemical reactions.
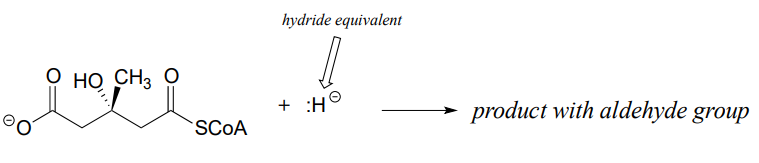
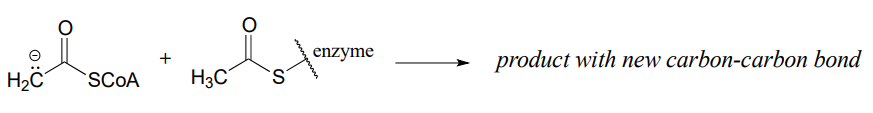
Predict the products of the following nucleophilic acyl substitution reactions, both of which are part of the biosynthesis of isoprenoid compounds such as cholesterol and lycopene:
- acetoacetyl CoA acetyltransferase reaction (enolate nucleophile)
- HMG-CoA reductase reaction (to repeat, the nucleophile here is not literally an isolated hydride ion, which would be a very unlikely species in a physiological environment. We will learn in chapter 16 what is actually going on, but for the time being, just predict the result of an acyl substitution reaction with a "hydride ion" nucleophile.)