29.6: Condensation Polymers
- Page ID
- 22397
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)There is a wide variety of condensation reactions that, in principle, can be used to form high polymers. However, high polymers can be obtained only in high-yield reactions, and this limitation severely restricts the number of condensation reactions having any practical importance. A specific example of an impractical reaction is the formation of poly-1,4-butanediol by reaction of 1,4-bromobutane with the disodium salt of the diol:
It is unlikely that this reaction would give useful yields of any very high polymer because \(E2\) elimination, involving the dibromide, would give a double-bond end group and prevent the chain from growing.
Polyesters
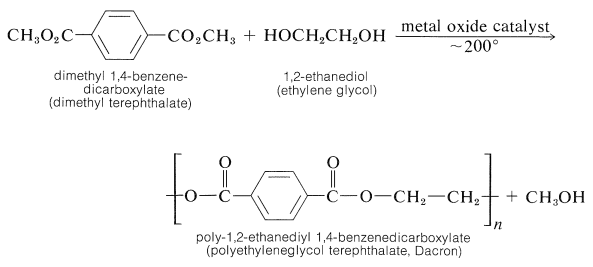
A variety of polyester-condensation polymers are made commercially. Ester interchange (Section 18-7A) appears to be the most useful reaction for preparation of linear polymers:
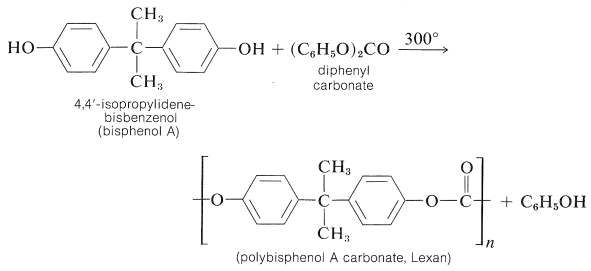
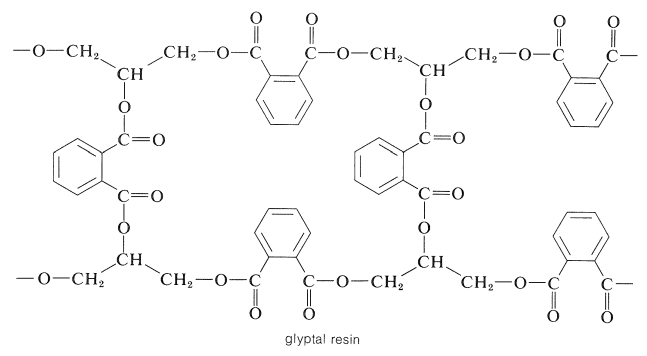
Thermosetting space-network polymers can be prepared through the reaction of polybasic acid anhydrides with polyhydric alcohols. A linear polymer is obtained with a bifunctional anhydride and a bifunctional alcohol, but if either reactant has three or more reactive sites, then formation of a three-dimensional polymer is possible. For example, 2 moles of 1,2,3-propanetriol (glycerol) can react with 3 moles of 1,2-benzenedicarboxylic anhydride (phthalic anhydride) to give a highly cross-linked resin, which usually is called a glyptal:
The first stage of the reaction involves preferential esterification of the primary hydroxyl groups with the anhydride to give
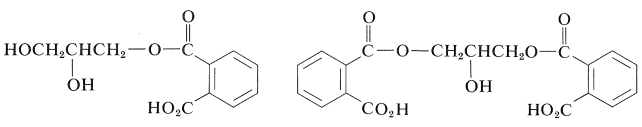
In the next stage, in the formation of the resin, direct esterification occurs slowly, particularly at the secondary hydroxyls. Normally, when the resin is used for surface coatings, esterification is carried only to the point where the polymer is not so cross-linked as to be insoluble. It then is applied to the surface in a solvent and baked until esterification is complete. The product is hard, infusible, and insoluble, being cross-linked to the point of being essentially one large molecule.
A wide variety of thermosetting polyester (alkyd) resins can be made by similar procedures. The following polybasic acids and anhydrides and polyhydric alcohols are among the other popular ingredients in alkyd formulations:
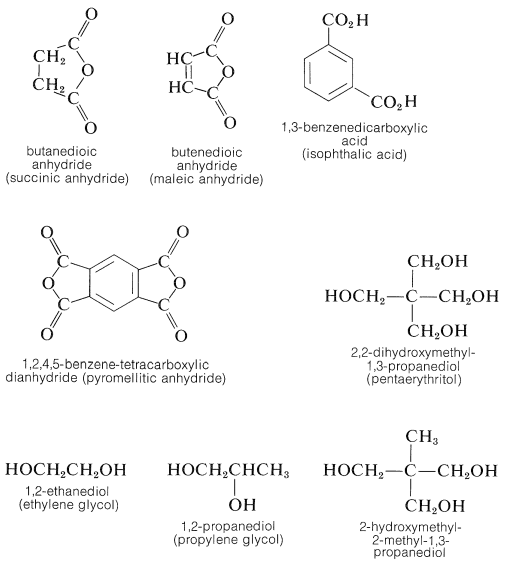
Articles in which glass fibers are imbedded to improve impact strength often are made by mixing the fibers with an ethenylbenzene (styrene) solution of a linear glycol (usually 1,2-propanediol)-butendioic anhydride polyester and then producing a cross-linked polymer between the styrene and the double bonds in the polyester chains by a peroxide-induced radical polymerization (Section 29-6E).
Nylons
A variety of polyamides can be made by heating diamines with dicarboxylic acids. The most generally useful of these is nylon 66, the designation 66 arising from the fact that it is made from the six-carbon diamine, 1,6-hexanediamine, and a six-carbon diacid, hexanedioic acid:
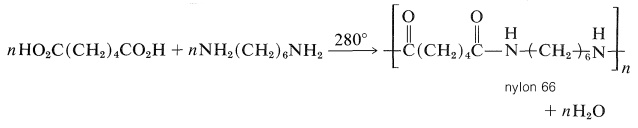
The polymer can be converted into fibers by extruding it above its melting point through spinnerettes, then cooling and drawing the resulting filaments. It also is used to make molded articles. Nylon 66 is exceptionally strong and abrasion resistant.
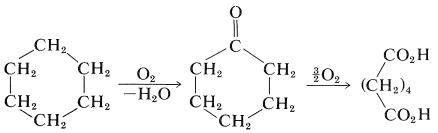
The starting materials for nylon 66 can be made in many ways. Apparently, the best route to hexanedioic acid is by air oxidation of cyclohexane by way of cyclohexanone:
1,6-Hexanediamine can be prepared in many ways. One is from 1,3-butadiene by the following steps:
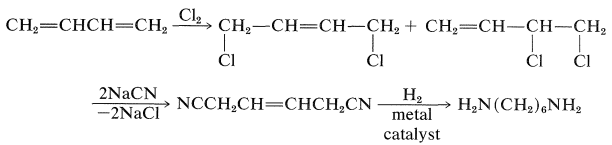
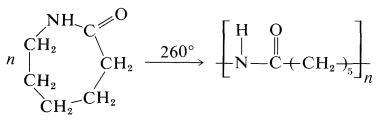
Nylon 6 can be prepared by polymerization of 1-aza-2-cycloheptanone (\(\varepsilon\)-caprolactam), obtained through the Beckmann rearrangement of cyclohexanone oxime (Section 24-3C):
Bakelite Resins
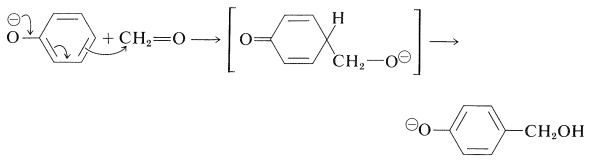
One of the oldest known thermosetting synthetic polymers is made by condensation of phenols with aldehydes using basic catalysts. The resins that are formed are known as Bakelites. The initial stage is the base-induced reaction of benzenol and methanal to give a (4-hydroxyphenyl)methanol, and this reaction closely resembles an aldol addition and can take place at either the 2- or the 4-position of the benzene ring:
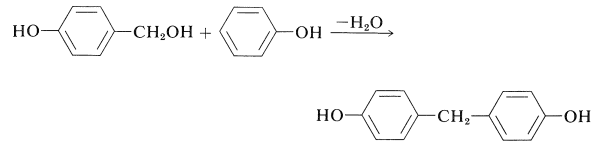
The next step in the condensation is formation of a bis(hydroxyphenyl)methane derivative, which for convenience is here taken to be the 4,4'-isomer:
This reaction is probably a Michael type of addition to a base-induced dehydration product of the (4-hydroxyphenyl)methanol:
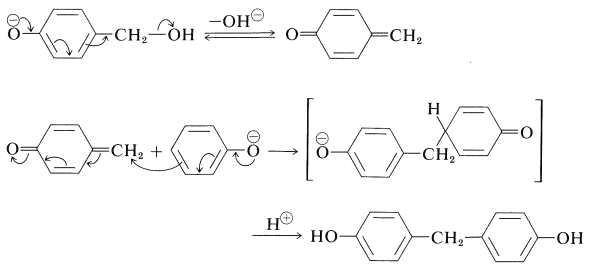
Continuation of these reactions at the 2-, 4-, and 6-positions of the benzenol leads to the cross-linked three-dimensional Bakelite resin:
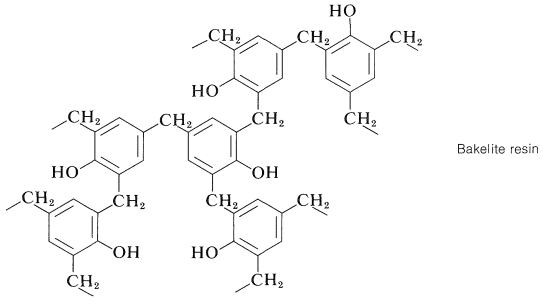
As with the alkyd resins (Section 29-5A), the initial polymerization of a Bakelite resin usually is carried to only a relatively low stage of completion. The low-melting prepolymer (called a resole) then is heated in a mold to give the final insoluble, infusible polymer.
Urea-Methanal and Melamine Resins
Syntheses of a number of polymers are based on condensation of methanal with amino compounds by mechanisms at least formally analogous to those involved in the preparation of Bakelite resins. The key reactions are:
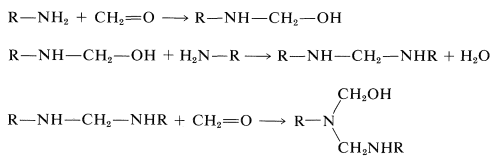
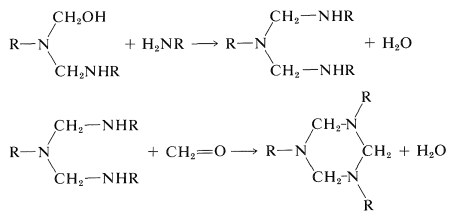
If the amino compound is urea, these types of reactions will lead to a three-dimensional polymer:
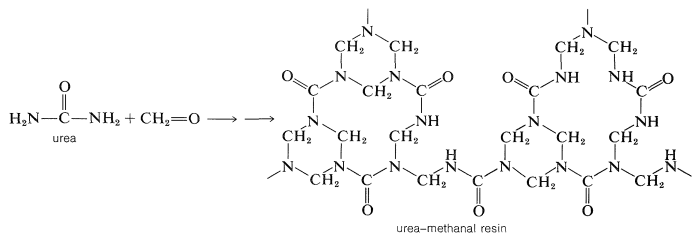
A similar polymer is made from 2,4,6-triamino-1,3,5-triazine (melamine) and methanal. It is used for plastic dishes under the name Melmac.
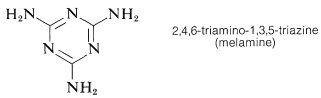
Epoxy Resins
A very useful group of adhesives and plastics is based on condensation polymers of bisphenol A and chloromethyloxacyclopropane (epichlorohydrin). The first step in the formation of epoxy resins is to form a prepolymer by condensation polymerization of the sodium salt of bisphenol A with the epoxide:
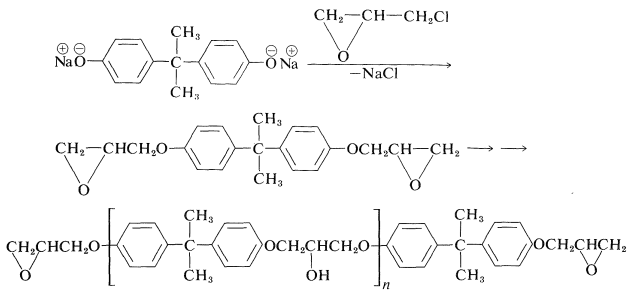
The formation of a prepolymer involves two different kinds of reactions. One is an \(S_\text{N}2\)-type displacement, and the other is oxide-ring opening of the product by attack of more bisphenol A. Usually, for practical purposes the degree of polymerization \(n\) of the prepolymer is small (5 to 12 units).
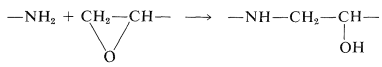
The epoxy prepolymer can be cured, that is, converted to a three-dimensional network, in several different ways. A trifunctional amine, such as \(\ce{NH_2CH_2CH_2NHCH_2CH_2NH_2}\), can be mixed in and will extend the chain of the polymer and form cross-links by reacting with the oxide rings:
Alternatively, a polybasic acid anhydride can be used to link the chains through combination with secondary alcohol functions and then the oxide rings.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


