25.14: Chemical Evolution
- Page ID
- 22370
A problem of great interest to those curious about the evolution of life concerns the origins of biological molecules. When and how were the molecules of life, such as proteins, nucleic acids, and polysaccharides, first synthesized?
In the course of geological history, there must have been a prebiotic period when organic compounds were formed and converted to complex molecules similar to those we encounter in living systems. The composition of the earth's atmosphere in prebiotic times was almost certainly very different from what it is today. Probably it was a reducing atmosphere consisting primarily of methane, ammonia, water, and because there was little or no free oxygen, there was no stratosphere ozone layer and little, if any, screening from the sun's ultraviolet radiation. Starting with \(\ce{CH_4}\), \(\ce{NH_3}\), and \(\ce{H_2O}\), it is plausible that photochemical processes would result in formation of hydrogen cyanide, \(\ce{HCN}\), and methanal, \(\ce{CH_2O}\), by reactions such as the following:
\[\ce{CH_4} + \ce{NH_3} \overset{h \nu}{\longrightarrow} \ce{HCN} + 3 \ce{H_2}\]
\[\ce{CH_4} + \ce{H_2O} \overset{h \nu}{\longrightarrow} \ce{CH_2=O} + 2 \ce{H_2}\]
Hydrogen cyanide and methanal are especially reasonable starting materials for the prebiotic synthesis of amino acids, purine and pyrimidine bases, ribose, and other sugars. Formation of glycine, for example, could have occurred by a Strecker synthesis (Section 25-6), whereby ammonia adds to methanal in the presence of \(\ce{HCN}\). Subsequent hydrolysis of the intermediate aminoethanenitrile would produce glycine:
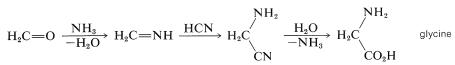
The plausibility of these reactions is strongly supported by the classic experiments of S. Miller (1953), who showed that a mixture of methane or ethane, ammonia, and water, on prolonged ultraviolet irradiation or exposure to an electric discharge, produced a wide range of compounds including racemic \(\alpha\)- and \(\beta\)-amino acids.
It is not difficult to visualize how sugars such as ribose may be formed. Methanal is known to be converted by bases through a series of aldol-type additions to a mixture of sugarlike molecules called "formose". Formation of racemic ribose along with its stereoisomers could occur as follows:
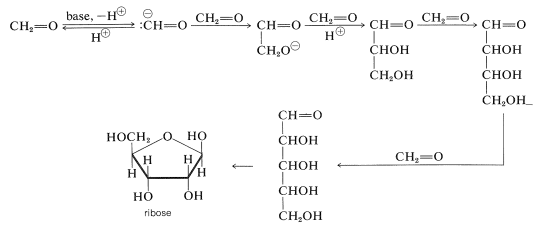
It is more difficult to conceive how the nucleic acid bases such as adenosine may be achieved in prebiotic syntheses. However, adenine, \(\ce{C_5H_5N_5}\), corresponds in composition to a pentamer of hydrogen cyanide and could result by way of a trimer of \(\ce{HCN}\), \(20\), and the adduct of ammonia with \(\ce{HCN}\), \(21\):
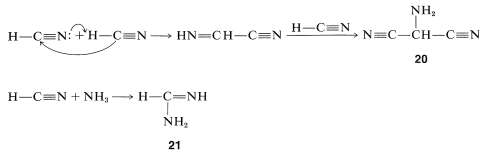
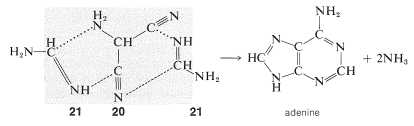
Without worrying about the mechanistic details of how adenine could be formed from \(20\) and \(21\), you can see that the adenine ring system is equivalent to \(20\) plus two \(21\) minus two \(\ce{NH_3}\):
How did these small prebiotic organic molecules grow into large polymeric substances such as peptides, RNA, and so on? It is important to recognize that by whatever reactions polymerization occurred, they had to be reactions that would occur in an essentially aqueous environment. This presents difficulties because condensation of amino acids to form peptides, or of nucleotides to form RNA or DNA, is not thermodynamically favorable in aqueous solution.
It is quite possible that primitive polypeptides were formed by the polymerization of aminoethanenitriles produced by the addition of ammonia and \(\ce{HCN}\) to methanal or other aldehydes. The resulting imino polymers certainly would hydrolyze to polypeptides:
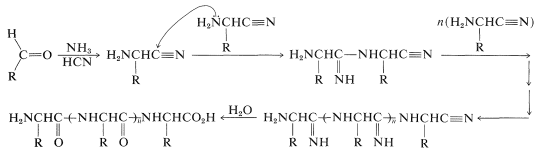
There are many other questions regarding the origin of the molecules of life for which we have only partial answers, or no answers at all.
It is difficult to imagine just how these molecules, once formed, somehow evolved further into the extraordinarily complex systems afforded by even the simplest bacterium able to utilize energy from the sun to support and reproduce itself. Nonetheless, synthetic peptides do coil and aggregate rather like natural proteins, and some also have shown catalytic activities characteristic of natural enzymes. One would hope that some kind of life would be found elsewhere in the solar system, the analysis of which would help us to better understand how life began on earth.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


