25.7: Aromatic Hydrocarbons
- Page ID
- 53999
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Friedrich Kekulé was a German chemist in the 1800s. Supposedly, he was thinking about the structure of benzene one night as he fell asleep. While asleep, he dreamed of a snake eating its own tail. He used this idea to propose the cyclic structure of benzene. Whether or not he actually had this dream has been debated ever since. Whatever really happened, the tale persists today.
Aromatic Hydrocarbons
Benzene is the parent compound of the large family of organic compounds known as aromatic compounds. Unlike cyclohexane, benzene only contains six hydrogen atoms, giving the impression that the ring is unsaturated, and that each carbon atom participates in one double bond. Two different structures with alternating single and double bonds around the ring can be written for benzene.
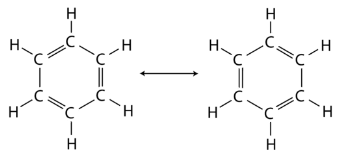
In benzene, the true bonding between carbon atoms is neither a single nor a double bond. Rather, all of the bonds are a hybrid of a single and double bond. In benzene, the pi bonding electrons are free to move completely around the ring. Delocalized electrons are electrons that are not confined to the bond between two atoms, but are instead allowed to move between three or more. The delocalization of the electrons in benzene can best be shown by showing benzene with a ring inside the hexagon, with the hydrogen atoms understood.
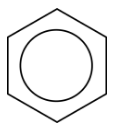
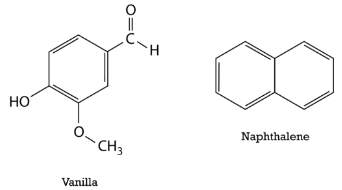
Delocalization of the electrons makes for a more stable molecule than a similar molecule that does not have delocalized electrons. Benzene is a more stable and less reactive compound than straight-chain hexenes. The \(sp^2\) hybridization of the carbon atoms results in a planar molecule, as opposed to the puckered structure of cyclohexane. Benzene rings are common in a great number of natural substances and biomolecules. The figure below shows the structural formulas for vanilla and naphthalene. Naphthalene is a chemical which is commonly used in mothballs.
Nomenclature of Aromatic Compounds
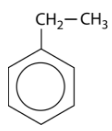
The simplest aromatic compounds are benzene rings with one substituent replacing one of the hydrogen atoms. If this substituent is an alkyl group, it is named first, followed in one word with "benzene". The molecule shown below is therefore called ethylbenzene.
Substituents can be groups other than alkyl groups. If a chlorine atom were substituted for a hydrogen, the name becomes chlorobenzene. An \(\ce{-NH_2}\) group is called an amino group, so the corresponding molecule is called aminobenzene, often referred to as an aniline. An \(\ce{-NO_2}\) group is called a nitro group, and so the third example below is nitrobenzene.
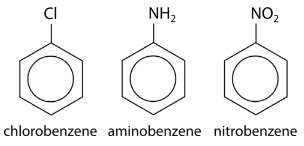
If more than one substituent is present, their location relative to one another can be indicated by numbering the positions on the benzene ring.
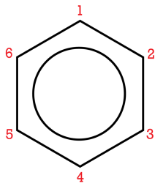
The number of the carbon location then precedes the name of the substituent in the overall name, with the numbers separated by a comma. As with branched alkanes, the system requires that the numbers be the lowest possible, and that prefixes be used for more than one of the same substituent. If there are different substituents, the first in alphabetical order is given the lower number and listed first. The structures below are called 1,2-dimethylbenzene and 1-ethyl-4-methylbenzene.
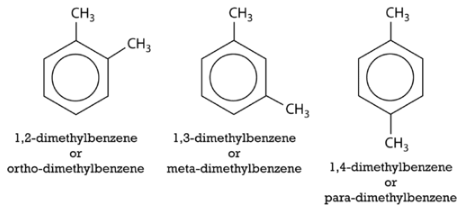
An alternate system for naming di-substituted benzene rings uses three different prefixes: ortho, meta, and para. If two groups are in the ortho position, they are on adjacent carbon atoms. The meta positioning refers to being in a 1,3 arrangement. The para positioning refers to being in a 1,4 arrangement. Shown below are the three possibilities for dimethylbenzene, also called xylene.
Lastly, a benzene ring missing one hydrogen atom \(\left( \ce{-C_6H_5} \right)\) can itself be considered the substituent on a longer chain of carbon atoms. That group is called a phenyl group and so the molecule below is called 2-phenylbutane.

Summary
- Benzene is the parent compound of the large family of organic compounds known as aromatic compounds.
- In benzene, the pi bonding electrons are free to move completely around the ring.
- Delocalized electrons are electrons that are not confined to the bond between two atoms, but are instead allowed to move between three or more.
- Nomenclature for benzene compounds is dictated.

