9.3: Oxidation of glucose -the glycolysis
- Page ID
- 433996
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Understand glycolysis and the essential reactions in this metabolic pathway responsible for glucose oxidation to two pyruvate molecules.
- Understand the reactions that pyruvate undergoes in the absence and presence of oxygen before entering the third stage of catabolism.
Glycolysis
Oxidation of glucose is the 2nd stage of the catabolism of carbohydrates. It happens in the cytoplasm of the cell. It is a metabolic pathway consisting of ten glycolysis reactions, as illustrated in Figure \(\PageIndex{1}\).
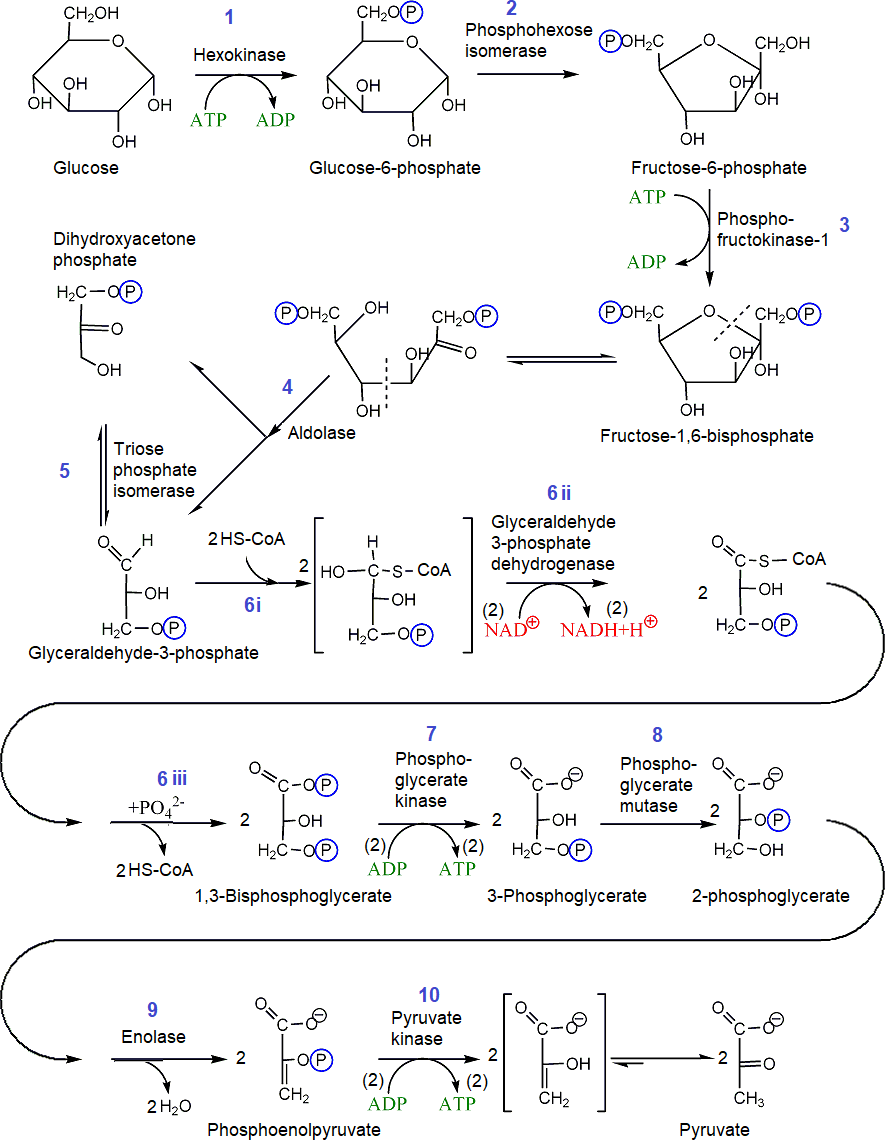
The ten reactions of glycolysis are the following (copyright: modified from a Public Domain resource at Wikipedia.)
1. Glucose is converted to glucose-6-phosphate by an SN2 reaction mechanism between a primary alcohol acting as a nucleophile and a \(\ce{P}\) of \(\ce{ATP}\) as an electrophile, where \(\ce{ADP}\) acts as a good leaving group, as shown below.

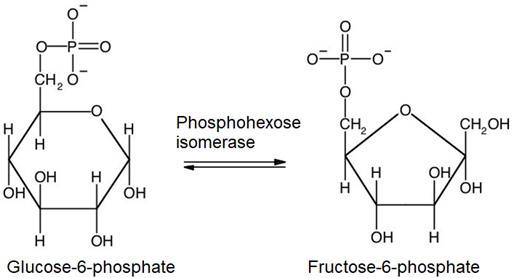
2. Glucose-6-phosphate, which is an aldohexose, isomerizes to fructose-6-phosphate, which is a ketohexose.
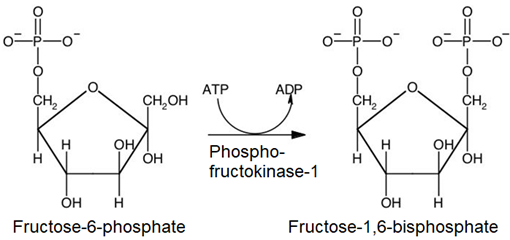
3. The same mechanism phosphorylates the primary alcohol group of fructose-6-phosphate as in the first step producing fructose-1,6-bisphosphate.
4. The open-chain form of fructose-1,6-bisphosphate, which is in equilibrium with the cyclic form, splits into two compounds, glyceraldehyde-3-phosphate and dihydroxyacetone phosphate.

5. This reaction is an isomerization reaction between glyceraldehyde-3-phosphate and its isomer dihydroxyacetone phosphate. The aldehyde or ketone in these two isomers tautomerizes to their enol forms, which either revert to the initial compound or to its isomers when the enol form changes back to an aldo or keto form.

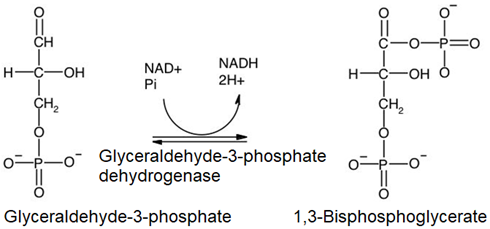
6. The aldehyde (\(\ce{-HC=O}\)) group of glyceraldehyde-3-phosphate is oxidized in three steps: activated by the addition of \(\ce{HS-CoA}\) (step 6i), followed by oxidation of \(\ce{-OH}\) to a carbonyl (\(\ce{C=O}\) group at the expense of reduction of \(\ce{NAD^{+}}\) to \(\ce{NADH}\) (step 6ii)); and finally displacement of \(\ce{HS-CoA}\) by a phosphate (\(\ce{-PO4^{2-}}\)) group (step 6iii). The overall reaction is the following.
The ketone group in dihydroxyacetone phosphate does not oxidize directly but converts to glyceraldehyde-3-phosphate due to the equilibrium creation#5. So, reaction#6 and reactions after this happen twice for each glucose molecule.
7. A phosphate group is transferred from 1,3-bisphosphoglycerate to ADP producing an ATP and a 3-phosphoglycerate.

Since this step happens twice for each glucose molecule processed, it compensates for the two ATP consumed, one in step#1 and the other in step#3.
8. 3-Phosphoglycerate is isomerized to 2-phosphoglycerate. In this step, the enzyme phosphoglycerate mutase first transfers its phosphate group to the \(\ce{-OH}\) at position#2 and then receives the phosphate from position#3 of the intermediate, resulting in the isomerization of 3-Phosphoglycerate to 2-phosphoglycerate.

9. 2-Phosphoglycerate is dehydrated, i.e., \(\ce{H2O}\) is eliminated form it, producing phosphoenolpyruvate.
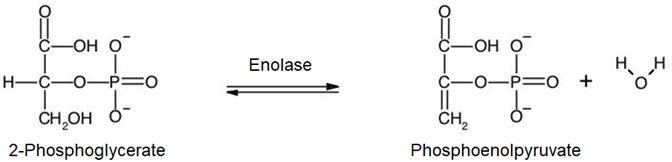
10. Phosphoenolpyruvate transfers its phosphate group to ADP producing pyruvate and ATP.

Since the reaction happens two times for each glucose, two ATP are produced in this step.
In summary, glycolysis is the oxidation of glucose without the need for oxygen, i.e., anaerobic oxidation, consisting of two phases. The first phase comprises reactions#1 to 5. It is the preparatory phase, which is also energy consuming phase in which a six \(\ce{C's}\) glucose (\(\ce{C6H12O6}\)) is phosphorylated twice at the expense of two ATP's and converts to two glyceraldehyde-3-phosphate molecules having three \(\ce{C's}\) each. The second phase, comprising reactions#6 to 10, is the payoff phase in which four ATP's are produced, i.e., two more than the ATP consumed in the first phase. Two \(\ce{NAD^{+}}\) are reduced to two \(\ce{NADH}\) along with the production of two pyruvates \(\ce{CH3COCOO^{-}}\) in the second phase, as shown in the following reaction.
\(\ce{\underbrace{C6H12O6}_{Glucose} + 2NAD^{+} + 2ADP + 2Pi -> 2\underbrace{CH3-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-O^{-}}_{Pyruvate} + 2NADH + 2ATP + 2H^{+} + 2H2O}\)
Fate of pyruvate
Two \(\ce{NAD^{+}}\) are reduced to two \(\ce{NADH}\) which need to be oxidized back to \(\ce{NAD^{+}}\) for the process to continue. It happens in different ways depending on whether sufficient oxygen is present, i.e., aerobic condition or aerobic respiration, or if there is no oxygen, i.e., anaerobic condition. The fate of pyruvate also depends on whether the condition is aerobic or anaerobic, as described below.
Aerobic condition
In aerobic conditions, \(\ce{NADH}\) is not oxidized at this stage, it is oxidized to \(\ce{NAD^{+}}\) at the expense of oxygen in mitochondria. The energy released by the oxidation of \(\ce{NADH}\) is used to produce more \(\ce{ATP's}\) in the third stage of catabolism. Pyruvate is also transferred to mitochondria and undergoes oxidation at the cost of \(\ce{NAD^{+}}\) reduced to \(\ce{NADH}\) through a series of reactions catalyzed by a complex of three enzymes and five coenzymes, collectively called the pyruvate dehydrogenase complex. The overall reaction is the decarboxylation of pyruvate, i.e., carbon dioxide (\(\ce{CO2}\)) eliminated. The acetyl (\(\ce{CH3-CO-}\)) group is transferred to \(\ce{HS-CoA}\) producing acetyl-\(\ce{CoA}\), as shown in the following reaction.
\(\ce{\underbrace{CH3-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-O^{-}}_{Pyruvate} + HS-CoA + NAD^{+} ->[\text{Pyruvate dehydrogenase complex}] \underbrace{CH3-\!\!-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-S-CoA}_{Acetyl\;CoA} + CO2 + NADH + H^{+}}\)
Acetyl-\(\ce{CoA}\) enters into the citric acid cycle, also called the Krebs cycle, in mitochondria, the third stage of catabolism.
The processing of pyruvate under aerobic conditions is also called oxidative decarboxylation or link reaction, which links glycolysis to the citric acid cycle, as explained in the video below.
Two pyruvates are produced per glycolysis of one glucose molecule. The overall reaction of one glucose molecule under aerobic conditions before entry into the citric acid cycle is the following.
\(\ce{\underbrace{C6H12O6}_{Glucose} + 2HS-CoA + 4NAD^{+} + 2ADP + 2Pi -> 2\underbrace{CH3-\!\!-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-S-CoA}_{Acetyl\;CoA} + 2CO2 + 4NADH + 2ATP + 4H^{+} + 2H2O}\)
There is a net gain of six energetic molecules: \(\ce{2ATP}\) and \(\ce{4NADH}\) from glycolysis and link reaction under aerobic conditions.
Anaerobic condition
 During vigorous exercises, as shown in the figure on the right, or strenuous physical work, oxygen depletes in muscles creating anaerobic (oxygen-free) conditions. Under anaerobic conditions, \(\ce{NADH}\) is oxidized to \(\ce{NAD^{+}}\) in the cytoplasm at the expense of the reduction of pyruvate to lactate, as shown in the reaction below.
During vigorous exercises, as shown in the figure on the right, or strenuous physical work, oxygen depletes in muscles creating anaerobic (oxygen-free) conditions. Under anaerobic conditions, \(\ce{NADH}\) is oxidized to \(\ce{NAD^{+}}\) in the cytoplasm at the expense of the reduction of pyruvate to lactate, as shown in the reaction below.
\(\ce{\underbrace{CH3-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-O^{-}}_{Pyruvate} + NADH + H^{+} ->[\text{Lactate dehydrogenase}] \underbrace{CH3-\!\!\!\!\!{\overset{\overset{\huge\;\;\;{OH}}|}{\enspace\;{CH}}}\!-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-O^{-}}_{Lactate} + NAD^{+}}\)
Overall, glycolysis of glucose under anaerobic conditions is the following reaction.
\(\ce{\underbrace{C6H12O6}_{Glucose} + 2ADP + 2Pi -> 2\underbrace{CH3-\!\!\!\!\!{\overset{\overset{\huge\;\;\;{OH}}|}{C}}\!\!\!-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-O^{-}}_{Lactate} + 2ATP}\)
The two \(\ce{NADH}\) produced in the glycolysis of one glucose are consumed in the anaerobic conversion of two pyruvates into two lactates.
- There is a net gain of two energetic molecules, i.e., \(\ce{2ATP}\) in glycolysis of one glucose followed by anaerobic conversion of two pyruvates into two lactates.
- The accumulation of lactate makes muscles tire and sour. The person keeps beating heavily after the exercise to pay the oxygen debt. Most of the lactate is transported to the liver, where it is re-oxidized to pyruvate.
Instead of converting pyruvate to lactate, as in humans and animals, yeast has an enzyme called pyruvate decarboxylase that decarboxylates pyruvate to acetaldehyde. Then \(\ce{NADH}\) is oxidized to \(\ce{NAD^{+}}\) at the expense of the reduction of acetaldehyde to ethanol in a process called fermentation, as shown in the reaction below.
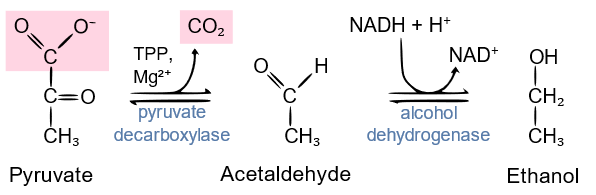
Copyright; User: Cwernert at en.Wikipedia, vectorized by User: GKFX., CC BY-SA 3.0, via Wikimedia Commons.
The overall reaction of glycolysis of glucose in the fermentation process by yeast.
\(\ce{\underbrace{C6H12O6}_{Glucose} + 2ADP + 2Pi -> 2\underbrace{CH3-CH2-OH}_{Ethanol} + 2CO2 + 2ATP}\)


