9.1: Basics of metabolism
- Page ID
- 433994
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Define metabolism, its subclasses: catabolism and anabolism, and stages of food catabolism.
- Understand the structure of a typical cell and mitochondrion -the sites of catabolism.
- Understand the basic structural features and functions of some common compounds involved in the catabolism of food, including ATP/ADP, NAD+/NADH, FAD/FADH2 pairs.
- Understand the basic structural features of enzymes that are involved in their catalytic activity.
What is metabolism
A large number of chemical reactions take place in living things almost all the time. Some of these reactions synthesize the substances living things need from the raw materials available. Usually, these reactions convert simple molecules or compounds to more complex molecules or compounds at the expense of energy. For example, photosynthesis converts carbon dioxide (\(\ce{CO2}\)) and water (\(\ce{H2O}\)) into glucose (\(\ce{C6H12O6}\)) by utilizing energy from sunlight.
\(\ce{6CO2 + 6H2O + Energy (2808 kJ/mol) -> C6H12O2}\)
Other reactions break down the complex molecules to release the energy needed as heat, to do work, to provide energy for synthetic reactions, to dispose of waste byproducts, etc. For example, the reverse of photosynthesis happens in the digestion of glucose.
\(\ce{6CO2 + 6H2O + Energy (2808 kJ/mol)C6H12O2 -> 6CO2 + 6H2O + Energy (2808 kJ/mol)}\)
 Metabolism is chemical reactions taking place in living things needed to sustain life.
Metabolism is chemical reactions taking place in living things needed to sustain life.
Metabolism can be subdivided into two categories of reactions.
- Catabolism is a set of chemical reactions in living things that breaks down molecules to release energy or obtain intermediates needed in other reactions.
- Anabolism, called biosynthesis, is a set of reactions in living things constructing molecules from smaller units.
The figure on the right summarizes metabolism and its sub-categories: catabolism and anabolism (Copyright: Linares-Pastén, J. A. (2018), CCBYSA 4, via Wikimedia Commons)
Metabolism usually happens through a series of interconnected chemical reactions called metabolic pathways. Metabolic pathways have a lot of similarities in different species. It indicates their common origin in the early stages of the evolution of species and their retention due to their efficiency. Some diseases, such as type II diabetes and cancer, disrupt normal metabolism. The difference in metabolic pathways from the normal allows scientists to find therapeutic interventions. Some of those metabolic pathways, particularly the catabolic pathways related to the digestion of food and its conversions to obtain energy, will be described in this chapter.
Stages of catabolism of food
 The catabolism of food starts from the digestion of food. It can be divided into three major stages, as illustrated in the figure on the right (Copyright: modified from Tim Vickers, vectorized by Fvasconcellos, Public domain, via Wikimedia Commons).
The catabolism of food starts from the digestion of food. It can be divided into three major stages, as illustrated in the figure on the right (Copyright: modified from Tim Vickers, vectorized by Fvasconcellos, Public domain, via Wikimedia Commons).
 Stage 1
Stage 1
It is mainly the hydrolysis of food molecules in the digestive system, shown in the figure on the left (Copyright: National Cancer Institute, Public domain, via Wikvia Wikimedians).
Polysaccharides are hydrolyzed to monosaccharides, fats are hydrolyzed to glycerol and fatty acids, and proteins are hydrolyzed to amino acids. The products of the stage 1 reactions diffuse into the bloodstream and are transported to cells, entering the next phase of food catabolism.
Stage 2
It happens in the cells, which is the degradation of monosaccharides, fatty acids, and amino acids. It yields smaller groups, usually a two-carbon acetyl group or, in the case of some amino acids, four-carbon carboxylate groups, which enter the next stage.
Stage 3
It happens in mitochondria in the cells. Stage 3 can be divided into three sub-stages described below.
Stage 3i: It is the citric acid cycle. In this stage, the two-carbon acetyl or four-carbon carboxylate groups are oxidized to carbon dioxide (\(\ce{CO2}\)) at the expense of the reduction of coenzymes \(\ce{NAD^+}\) and \(\ce{FAD}\) to \(\ce{NADH}\) and \(\ce{FADH2}\).
Stage 3ii: It is electron transport where coenzymes \(\ce{NADH}\) and \(\ce{FADH2}\) are reduced back to their oxidized forms \(\ce{NAD^+}\) and \(\ce{FAD}\) at the expense of reduction of oxygen (\(\ce{O2}\)) into water (\(\ce{H2O}\)) via electron transport process with release of energy.
Stage 3iii: It is an oxidative phosphorylation process where the energy released during the electron transport stage is used to synthesize adenosine triphosphate (ATP), which is a high-energy molecule from adenosine diphosphate (ADP), and phosphate (Pi), which are lower-energy molecules. The energy is temporarily stored in the form of ATP and released wherever it may be needed by the reverse reaction, i.e., conversion of ATP into ADP and Pi.
Cell structure related to food catabolism
 Stages 2 and 3 of food catabolism happen in the cells. A cell membrane surrounds a typical animal cell. Organelles are organized or specialized structures within the cells. A typical animal cell is illustrated in the right figure with some organelles labeled (Copyright; National Human Genome Research Institute, Public domain).
Stages 2 and 3 of food catabolism happen in the cells. A cell membrane surrounds a typical animal cell. Organelles are organized or specialized structures within the cells. A typical animal cell is illustrated in the right figure with some organelles labeled (Copyright; National Human Genome Research Institute, Public domain).
The nucleus in the cell contains hereditary material, i.e., DNA. The space between the cell membrane and the nucleus is called the cytoplasm. The cytosol is the fluid part of the cytoplasm containing an aqueous solution of electrolytes and enzymes that catalyze many of the cell's chemical reactions. Within the cytoplasm are organelles that perform specialized functions. For example, ribosomes are the sites for protein synthesis, and mitochondria are the cell's energy factory where stage 3 of food catabolism occurs.
Mitochondrion
 The mitochondrion (plural mitochondria) has an outer membrane and inner membrane and an inter-membrane space between the two, as shown in the figure on the left (Copyright; LadyofHats, Public domain, via Wikimedia Commons). The fluid section surrounded by the inner membrane is called the matrix. The enzymes that catalyze the chemical reactions of stage 3 of food catabolism are located in the matrix and along the inner membrane. These reactions ultimately convert the food molecules into \(\ce{CO2}\), \(\ce{H2O}\), and energy. The enzymes that catalyze stage 4 reactions that use this energy to produce high-energy ATP also occur in the matrix along the inner membrane.
The mitochondrion (plural mitochondria) has an outer membrane and inner membrane and an inter-membrane space between the two, as shown in the figure on the left (Copyright; LadyofHats, Public domain, via Wikimedia Commons). The fluid section surrounded by the inner membrane is called the matrix. The enzymes that catalyze the chemical reactions of stage 3 of food catabolism are located in the matrix and along the inner membrane. These reactions ultimately convert the food molecules into \(\ce{CO2}\), \(\ce{H2O}\), and energy. The enzymes that catalyze stage 4 reactions that use this energy to produce high-energy ATP also occur in the matrix along the inner membrane.
The principal compounds involved in the common metabolic pathways
The principal compounds involved in the common metabolic pathways are adenosine triphosphate (\(\ce{ATP}\)), which is the agent for the temporary storage of energy and transfer of phosphate (\(\ce{PO4^{3-}}\) or \(\ce{Pi}\)) group; nicotinamide adenine dinucleotide (\(\ce{NAD^+}\)) and flavin adenine dinucleotide (\(\ce{FAD}\)) which are agents for the transfer of electrons during the biological oxidation-reduction sections; and coenzyme A (\(\ce{CoA}\)) which is the agent for the transfer of acetyl (\(\ce{CH3CO{-}}\)) group. These compounds are described here.
ATP -the energy currency and agent for the transfer of phosphate groups
Adenosine triphosphate (\(\ce{ATP}\)) is a nucleotide composed of adenine base bonded to ribose sugar by an N-glycosidic bond and triphosphate bonded to ribose by an ester bond, as shown in Figure \(\PageIndex{1}\). The N-glycoside of adenine with ribose is a nucleoside, i.e., adenosine. When adenosine is bonded to diphosphate, it makes adenosine diphosphate (\(\ce{ADP}\)).

Hydrolysis of \(\ce{ATP}\) splits one phosphate ( \(\ce{PO4^{3-}}\) or \(\ce{Pi}\)) and convert it to \(\ce{ADP}\) with the release of 30.5 kJ/mol energy.
\(\ce{ATP + H2O -> ADP + Pi}\) \(\delta\)H = -30.5 kJ/mol
This energy is used in the processes that require energy, such as muscle contraction, nerve signal conduction, and biosynthesis. Catabolism of food releases energy temporarily stored in the form of \(\ce{ATP}\) by reversing the above reaction.
\(\ce{ADP + Pi -> ATP + H2O}\) \(\delta\)H = +30.5 kJ/mol
The cycle of reactions between \(\ce{ATP}\) and \(\ce{ADP}\) shown in Figure \(\PageIndex{1}\) happen rapidly, producing one to two million \(\ce{ATP's}\) per second. An average human body produces \(\ce{ATP}\) about equal to the human body mass per day, but it contains about 1 g of \(\ce{ATP}\) at a time.
\(\ce{ADP}\) can also hydrolyze with water releasing energy and converting to adenosine monophosphate (\(\ce{AMP}\)
\(\ce{ADP + H2O -> AMP + Pi}\) \(\delta\)H = -30.5 kJ/mol
\(\ce{ATP}\) is also a phosphorylation agent in metabolic reactions. For example, D-glucose is phosphorylated by the reaction shown below.

In summary the main roles of \(\ce{ATP}\)/\(\ce{ADP}\) pair are:
- energy transfer, i.e., releases energy when \(\ce{ATP}\) converts not \(\ce{ADP}\) + \(\ce{P_{i}}\) and absorbs energy in the reverse reaction,
- transfer of phosphate (\(\ce{P_{i}}\)), i.e., releases phospahte when \(\ce{ATP}\) converts not \(\ce{ADP}\) + \(\ce{P_{i}}\) and consumes phosphate in the reverse reaction.
\(\ce{NAD^+}\) and \(\ce{FAD}\) -agents for the transform of electrons
Oxidation and reduction
Oxidation is:
- loss of electron (\(\ce{e^-}\)),
- gain of oxygen (\(\ce{O}\)), or
- loss of hydrogen (\(\ce{H}\)).
Reduction is:
- gain of electron (\(\ce{e^-}\)),
- loss of oxygen (\(\ce{O}\)), or
- gain of hydrogen (\(\ce{H}\)).
Generally, oxidation reactions release energy, and reduction reactions gain energy.
Oxidation and reductions are coupled, i.e., if one reagent is oxidized, another is reduced simultaneously. For example, in the reaction shown below, pyruvate is oxidized by losing hydrogen coenzyme \(\ce{NAD^{+}}\) is reduced by gaining hydrogen. So, oxidation-reduction couples are collectively called redox reactions.
\(\ce{\underbrace{CH3-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-O^{-}}_{Pyruvate} + NADH + H^{+} <->[\text{Lactate dehydrogenase}] \underbrace{CH3-\!\!\!\!\!{\overset{\overset{\huge\;\;\;{OH}}|}{\enspace\;{CH}}}\!-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-O^{-}}_{Lactate} + NAD^{+}}\)
Oxygen may be added to the compound, i.e., make a single or double bond with the substrate, or it may change from a single bond to a double bond in the substrate, e.g., \(\ce{-C-O}\) to \(\ce{-C=O}\), both ways it is oxidation. That is, an increase in the bond with oxygen is oxidation.
In metabolic reactions, \(\ce{H}\) is represented as a proton \(\ce{H^+}\) and an electron \(\ce{e^-}\). So, the addition of \(\ce{H^+}\) + \(\ce{e^-}\) is reduction, and their removal is oxidation. Usually, \(\ce{2H^{+}}\) + \(\ce{2e^{-}}\) are transferred from or to a coenzyme in metabolic reactions.
If bonds with hydrogen added or removed are counterbalanced by bonds with oxygen removed or added, the overall reaction is not a redox. For example, an alkene's hydration, shown below, is not a redox reaction.
\(\ce{CH3CH2CH=CHCH2CH3 + H2O ->[H2SO4] CH3CH2CH2-\!\!\!\!\!{\overset{\overset{\huge\;\;\;{OH}}|}{\enspace\;{CH}}CH2CH3}}\)
Oxidation and reduction are not limited to making or breaking a bond with oxygen or hydrogen; broader definitions are the following.
Adding a bond with a more electronegative atom is oxidation, and its removal is reduction. The opposite is true for a less electronegative atom, i.e., The addition of a bond with a less electronegative atom is reduction, and its removal is oxidation. For example, conversion of \(\ce{R-\!\!\!\!\!{\overset{\overset{\huge\;\;\;{OH}}|}{\enspace\;{CH}}-R'}}\) into \(\ce{R-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-R'}\) replaces bond on \(\ce{C}\) from less electronegative \(\ce{H}\) with more electronegative \(\ce{O}\) is oxidation. Similarly, conversion of \(\ce{R-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-CH2R'}\) into \(\ce{R-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-SCH2R'}\) replaces bond on \(\ce{C}\) from a \(\ce{C}\) with more electronegative \(\ce{S}\) is oxidation. The reverse of these is reductions.
Nicotinamide adenine dinucleotide (\(\ce{NAD^{+}}\))
Nicotinamide adenine dinucleotide is a coenzyme composed of two nucleotides, an adenosine diphosphate (ADP) and a second nucleotide in which the nitrogen base is nicotinamide provided by vitamin niacin. The two nucleotides are linked by diphosphate linkage, as shown in Figure \(\PageIndex{2}\). The oxidized form is represented as \(\ce{NAD^{+}}\) and its reduced form is represented as \(\ce{NADH}\). The \(\ce{NAD^{+}}\) is reduced to \(\ce{NADH}\) by reacting with two hydrogen (\(\ce{2H^{+}}\) + \(\ce{2e^{-}}\)) leaving one \(\ce{H^{+}}\) in the products, as shown in Figure \(\PageIndex{2}\). Oxidation of \(\ce{NADH}\) to \(\ce{NAD^{+}}\) is the reverse reaction.

The \(\ce{NADH}\)/\(\ce{NAD^{+}}\) redox is coupled with \(\ce{\underbrace{R-\!\!\!\!\!{\overset{\overset{\huge\;\;\;{OH}}|}{\enspace\;{CH}}}\!-R'}}_{Alcohol}\) / \(\ce{\underbrace{R-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-R'}}_{Carbonyl}\), redox reactions or it involves carbonyl (\(\ce{C=O}\)) group in food catabolism .
An example of an oxidation reaction in metabolism is the oxidation of the alcohols (\(\ce{-OH}\)) group to carbonyl (\(\ce{C=O}\)) group. For example, ethanol is oxidized to ethanal in the liver at the expense of reduction of \(\ce{NAD^{+}}\) to \(\ce{NADH}\) as shown below.

Flavin adenine dinucleotide (\(\ce{FAD}\))
Flavin adenine dinucleotide is a coenzyme composed of two nucleotides, an adenosine diphosphate (ADP) and a second nucleotide which is riboflavin (vitamin B2). Riboflavin is composed of flavin and ribitol, which is a sugar alcohol. The two nucleotides are linked by an ester linkage between ribitol and diphosphate, as shown in Figure \(\PageIndex{3}\). The oxidized form is represented as \(\ce{FAD}\), and its reduced form is represented as \(\ce{FADH_{2}}\). The oxidation-reduction happens in the \(\ce{N}\) containing rings of the flavin part. The \(\ce{FAD}\) is reduced to \(\ce{FADH_{2}}\) by reacting with two hydrogen (\(\ce{2H^{+}}\) + \(\ce{2e^{-}}\)), as shown in Figure \(\PageIndex{2}\). Oxidation of \(\ce{FADH_{2}}\) to \(\ce{FAD}\) is the reverse reaction.

The \(\ce{FADH_{2}}\)/\(\ce{FAD}\) redox is coupled with \(\ce{\underbrace{RR'\!\!\!\!\!{\overset{\;\;\;\overset{\huge\;{H}}|}{\enspace\;{C}}}\!-\!\!\!\!\!{\overset{\;\;\;\overset{\huge\;{H}}|}{\enspace\;{C}}}\!RR'}}_{Single bond}\) / \(\ce{\underbrace{RR'C=CRR'}_{Double bond}}\), redox reactions, i.e., it involves \(\ce{C=C}\) bond in food catabolism.
An example is the oxidation of a \(\ce{C-C}\) bond to \(\ce{C=C}\) bond of fumarate at the expense of reduction of \(\ce{FAD}\) to \(\ce{FADH_{2}}\) as shown below.

Another example is the following step in the \(\beta\)-oxidation of fatty acids catalyzed by acyl-\(\ce{CoA}\) dehydrogenase.
Coenzyme A (\(\ce{CoA}\)) -the agent for transfer of acetyl (\(\ce{CH3CO{-}}\)) group
Coenzyme A (\(\ce{CoA}\)) is composed of several components, as illustrated in Figure \(\PageIndex{4}\).

The reactive part of \(\ce{CoA}\) is the thiol (\(\ce{-SH}\)) group which forms a high-energy thioester bond with acyl groups. Free coenzyme is usually represented as \(\ce{HS-CoA}\), and when it is as a thioester of the acyl group, it is represented as \(\ce{Ac-S-CoA}\), where \(\ce{Ac{-}}\) is an acyl group. For example, pyruvate transfers its acetyl (\(\ce{CH3CO{-}}\)) groups to \(\ce{HS-CoA}\) in the following reaction, which is a step in the catabolism of carbohydrates.

The \(\ce{-S-CoA}\) group in \(\ce{Ac-S-CoA}\) is a good leaving group that easily transfers the acyl group to other compounds during biosynthesis. For example, the acetyl group is transferred from \(\ce{Ac-S-CoA}\) to an acyl carrier protein (ACP) which is a step in the synthesis of fatty acids.

Nearly all metabolic reactions in living things are catalyzed by enzymes. Unlike chemical reactions in laboratories where different solvents, extreme temperature, and pressure conditions can be applied, and strong acids or bases can be used to catalyze the reactions, biomedical reactions in living things must occur under physiological conditions. Enzymes are specialized catalysts that catalyze the reactions under physiological conditions. The major factors responsible for the catalytic activity of the enzymes are the following.
- Enzymes have functional groups in their active sites that act as handles to bind with and lock the substrate in a proper orientation such that the reagent can easily approach the reactive site on the substrate. This way, any steric hindrance to the reaction is minimized.
- Enzymes and their co-factors also usually neutralize the ionic groups on the substrate that otherwise repel the charged or partially charged electrophile or nucleophile from approaching the substrate. This way, the electrostatic factors hindering the reaction are minimized.
- Enzymes usually have functional groups in their active sites that catalyze the reaction of the substrate by activating it as a nucleophile, electrophile, acid, or base catalyst.
The following two examples from glucose catabolism illustrate the factors mentioned above.
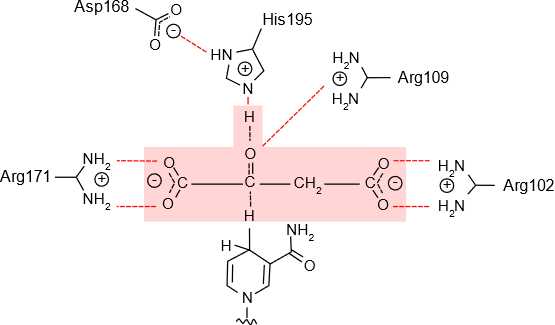
The enzyme malate dehydrogenase catalyzes the oxidation of malate to oxaloacetate in the citric acid cycle. The substrate is bound by the enzyme in such a way that i) the charge of its two carboxylates (\(\ce(-COO^{-}}\)) groups are neutralized by arginine side chains of the enzyme, ii) histidine side acts as a base catalyst and removes the proton from the \(\ce{-OH}\) group of the substrate, and at the same time, iii) the \(\ce{H}\) on the \(\ce{C}\) carrying the \(\ce{-OH}\) group is exposed to nicotine amide ring of \(\ce{NAD^{+}}\) that removes the proton, as illustrated in Figure \(\PageIndex{1}\),
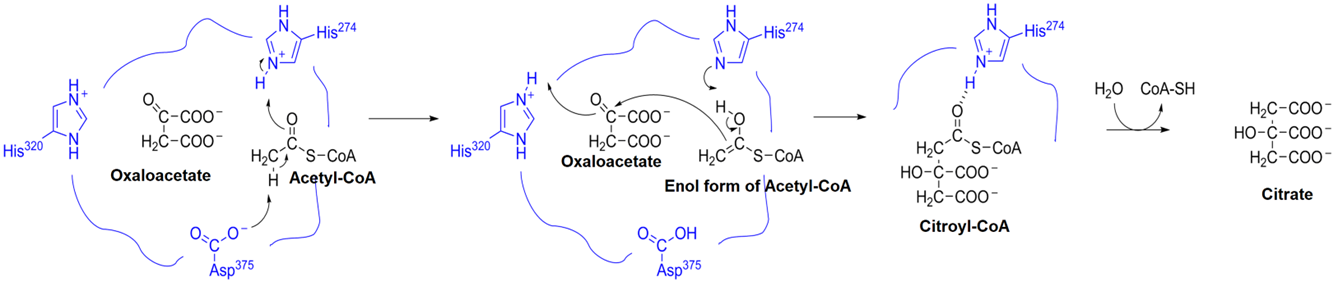
Another example is the formation of citrate from oxaloacetate and acetyl-\(\ce{CoA}\) in the citric acid cycle, catalyzed by the enzyme citrate synthase as illustrated in Figure \(\PageIndex{2}\).
- The substrate is bonded by the enzyme in such a way that the two substrates are at a bonding distance. The \(\ce{-CH3}\) hydrogens of acetyl-\(\ce{CoA}\) are acidic protons due to being \(\alpha\) to the carbonyl group. The asparagine side chain acts as a base catalyst and removes the acidic proton from \(\ce{-CH3}\) group of acetyl-\(\ce{CoA}\). The proton transfer to asparagine is facilitated by another proton transfer from histidine (His274) to the \(\ce{C=O}\) group converting it into its enol form -a nucleophile.
- The enol returns its proton to the histidine side chain in the second step activating the \(\ce{C=C}\) bond of the enol for a nucleophilic attack on the electrophilic ketone (\(\ce{C=O}\) group of oxaloacetate substrate, in the second step. This step is facilitated by the transfer of another proton from another histidine ((His320), the (\(\ce{C=O}\)) group involved in the reaction. The result is the conversion of the \(\ce{C=O}\) group into an (\(\ce{-O-H}\) group of citroyl-\(\ce{CoA}\) ubterneduate.
- Citroyl-\(\ce{CoA}\) intermediate is hydrogen-bonded to a histidine (His274). This hydrogen bond activates the thioester group for a hydrolysis reaction. Water molecule hydrolyzes the thioester group releasing the citrate product from the enzyme in this reaction step.
Nearly every metabolic reaction is catalyzed by an enzyme in a similar way as described above.


