5.7: Polysaccharides
- Page ID
- 423695
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Learn the structures and some characteristics of polysaccharides, including starches, cellulose, and chitin.
Polysaccharides are long polymers from ten to thousands of monosaccharides joined by glycosidic linkages. The most abundant polysaccharides are starch, glycogen, cellulose, and chitin. Except for chitin, all others are composed of D-glucose. Chitin is composed of a modified form of glucose. These are described next.
Starch
Starch is a storage form of D-glucose in plants. It is found in potatoes, beans, rice, wheat, and other grains and roots, as illustrated in Figure \(\PageIndex{1}\). Starch is a mixture of two forms, 20% to 25% amylose and 75 to 80% amylopectin.

Amylose
Amylose is an unbranched chain of up to 4,000 D-glucose units joined by \(\alpha\)-1,4-glycosidic linkage, as shown below.
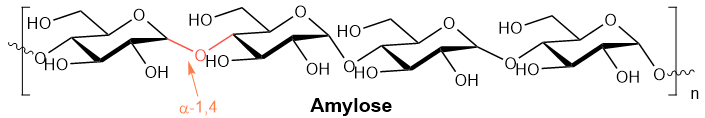
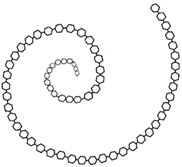
The orientation of bonds with \(\alpha\)-1,4-glycosidic linkage makes the amylose chain exist in a helical structure, as shown in Figure \(\PageIndex{2}\), that allows water molecules to access and establish hydrogen bonds with \(\ce{-OH}\) groups. Starch is a water-soluble polymer. It can dissolve in water due to extensive hydrogen bonding with the water molecules when mixed with water.

Amylopectin
Amylopectin is a branched chain polymer of D-glucose, as shown below.
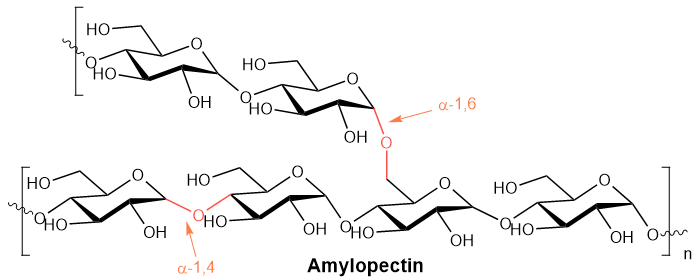
The main chain comprises 10,000 D-glucose units joined by \(\alpha\)-1,4-glycosidic linkage. The main chain exists in the form of a helix. Branches originate at every 24 to 30 unit intervals on the main chain connected to the main chain by a \(\alpha\)-1,6-glycosidic linkage, as shown in Figure \(\PageIndex{2}\). This helix with branches going outwards makes amylopectin more accessible to water for hydrogen bonding and easier to digest.
Glycogen
Glycogen is an energy-storage polysaccharide in animals with the same structure as amylopectin. it has up to 106 D-glucose units joined by \(\alpha\)-1,4-glycosidic linkages and branching through \(\alpha\)-1,6-glycosidic linkages. The main difference from amylopectin is that glycogen has more frequent branching at 10 to 15 D-glucose units interval, as illustrated in Figure \(\PageIndex{3}\). More branching makes it more soluble in water and easier to hydrolyze multiple D-glucose units from the ends of branches, when the body needs D-glucose.

Cellulose
Cellulose is the most abundant polysaccharide in nature that makes up about 50% of the cell wall of plant cells. Cotton, shown in Figure \(\PageIndex{4}\), is almost pure cellulose.

Cellulose is a linear polysaccharide of about 2200 D-glucose units joined by \(\beta\)-1,4-glycosidic linkage, as shown below.

The \(\beta\)-1,4-glycosidic linkage allowes glucose units to adopt a linear structure with intra-molecule hydrogen bonding. The linear polymer packs nicely with inter-molecular hydrogen bonding and the London-dispersion forces, as shown in Figure \(\PageIndex{5}\). It gives cellulose its mechanical strength and makes it insoluble in water. Due to the extensive inter- and intra-molecular hydrogen bonding within cellulose, water can not establish enough hydrogen-bonds to dissolve it.

Animals have \(\alpha\)-glucosidase enzymes that allow them to hydrolyze starch and glycogen to D-glucose, but they do not have \(\beta\)-glucosidase enzymes needed to hydrolyze cellulose. Grazing animals and termite host bacteria in their stomach that have the \(\beta\)-glucosidase enzymes. Therefore, grazing animals and termites can digest cellulose.
Chitin
Chitin is the second most abundant polysaccharide, second only to cellulose. It is found in the exoskeleton of crustaceans and insects, as shown in Figure \(\PageIndex{6}\).

Chitin has the same structure as cellulose except that it is composed of N-acetylglucosamine, an amide derivative of D-glucose, in place of D-glucose in cellulose, as illustrated below Figure \(\PageIndex{7}\).



