4.5: Nucleophilic acyl substitution reactions
- Page ID
- 416448
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Understand nucleophilic acyl substitution mechanisms, and factors that affect them.
- Apply nucleophilic acyl substitution to reactions of acid halides, anhydrides, carboxylic acids, esters, and amides.
- Wright the reactions with reagents to convert carboxylic acids into acid halides and phosphate esters in living things.
- Apply nucleophilic acyl substitution reactions to synthesize condensation polymers.
What is a nucleophilic acyl substitution reaction?
A carbon double bonded with oxygen, i.e., \(\ce{-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-}\) is a carbonyl group. The \(\ce{C}\) of the carbonyl group is bonded to two other groups. If one of the group bonded to the carbonyl \(\ce{C}\) is an alkyl (\(\ce{R{-}}\)), or hydrogen (\(\ce{H{-}}\)), it become an acyl group, i.e., \(\ce{R-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-}\). The acyl group has a polar double bond, i.e., \(\ce{R-\!\!{\overset{\overset{\huge\enspace\!{\overset{\Large{\delta{-}}}{O}}}|\!\!|\enspace}{\overset{\delta{+}}{C}}}\!\!-}\) where the carbonyl \(\ce{C}\) is partial positive (\(\delta{+}\)), i.e., it is an electrophile. If the acyl group is attached to a nucleophile that can act as a leaving group (\(\ce{-Lv}\)), a stronger nucleophile (\(\ce{Nu^{-}}\)) can substitute it from the acyl group in reactions called nucleophilic acyl substitution reactions, as shown below in a generalized reaction.
\[\ce{Nu^{-} + R-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-Lv -> R-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-Nu + Lv^{-}}\nonumber \]
Mechanisms of nucleophilic acyl substitution reactions
In nucleophilic acyl substitution reactions, there are two reactants, the nucleophile and the acyl group containing substrate. Nucleophiles can exist in anionic base form \(\ce{Nu^{-}}\) in a basic medium and neutral acid form \(\ce{HNu}\) in a neutral or acidic medium. The anionic form is a better nucleophile than its neutral acid form. Similarly, the substrate can exist as neutral \(\ce{R-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-Lv}\) in neutral or basic medium or as protonated \(\ce{R-\!\!\!\!\!\!{\overset{\overset{\huge\enspace\enspace{\overset{\Large{+}}{O}H}}|\!\!\!\!\!\!\!|\enspace\enspace}{\overset{\delta{+}}{C}}}\!\!\!\!\!\!-\overset{\delta{-}}{Lv}}\) form in an acidic medium. The protonated form has more \(\delta{+}\) charge and is a better electrophile. The anionic nucleophile cannot coexist with protonated substrate, because of their acid-base reaction. The other three combinations, i.e., basic nucleophile + neutral substrate, neutral nucleophile + protonated substrate, and neutral nucleophile + neutral substrate, can coexist as described below.
Base-promoted mechanism
The nucleophile in its more reactive basic form \(\ce{Nu^{-}}\) and neutral substrate \(\ce{R-\!\!{\overset{\overset{\huge\enspace\!{\overset{\Large{\delta{-}}}{O}}}|\!\!|\enspace}{\overset{\delta{+}}{C}}}\!\!-}\) can coexist in a basic medium. The nucleophile \(\ce{Nu^{-}}\) attacks the electrophilic carbonyl \(\ce{\overset{\delta{+}}{C}}\) and, simultaneously, the \(\ce{\overset{\delta{+}}{C}}\) breaks the weakest \(\pi\)-bond, as shown in step#1 of the mechanism below. The electrophilic \(\ce{C}\) changes hybridization from sp2 to sp3, i.e., a tetrahedral intermediate.

The nucleophilic \(\ce{O^{-}}\) created in the first step attacks the \(\ce{\overset{\delta{+}}{C}}\) to re-establish the \(\pi\)-bond, and either \(\ce{\overset{\delta{+}}{C}{-}\overset{\delta{-}}{Nu}}\) or \(\ce{\overset{\delta{+}}{C}{-}\overset{\delta{-}}{Lv}}\)-bond breaks. This step is also called the collapse of the tetrahedral intermediate. Breakage of \(\ce{\overset{\delta{+}}{C}{-}\overset{\delta{-}}{Nu}}\)-bond reverses the first step and breakage of \(\ce{\overset{\delta{+}}{C}{-}\overset{\delta{-}}{Lv}}\)-bond leads to the products of step#2. Later will likely happen when the \(\ce{-Lv}\) is a good leaving group. The second step is usually not reversible because \(\ce{Lv^{-}}\) is usually a poor nucleophile. The leaving group ultimately picks up a proton from any acid molecule (\(\ce{HB^{+}}\)) in the medium, as shown in step#3.
The rate of reaction is increased by converting the neutral or acid form of the nucleophile (\(\ce{HNu}\)) to its more reactive conjugate base form (\(\ce{Nu^{-}}\)) in the basic medium in this mechanism. Therefore, it is called base promoted nucleophilic acyl substitution mechanism.
Acid-catalyzed mechanism
In this mechanism, the nucleophile is in less reactive neutral \(\ce{HNu}\) form, but the substrate is in a more reactive protonated \(\ce{R-\!\!\!\!\!\!{\overset{\overset{\huge\enspace\enspace{\overset{\Large{+}}{O}H}}|\!\!\!\!\!\!\!|\enspace\enspace}{\overset{\delta{+}}{C}}}\!\!\!\!\!\!-\overset{\delta{-}}{Lv}}\) form, in an acidic medium. Acid in the medium protonate the carbonyl \(\ce{O}\) in step#1, as shown in the mechanics below.

Nucleophile \(\ce{HNu}\) attacks the protonated electrophilic carbonyl \(\ce{\overset{\delta{+}}{C}}\) and, simultaneously, the \(\pi\)-bond breaks in step#2, leading to a tetrahedral intermediate-I. The neutral nucleophile becomes +ve charged after donating its lone pair of electrons. It carries an acid proton. Although the medium is acidic in this case, it is usually amphoteric, with some basic groups still present. The incoming nucleophile donates its acidic proton to any basic molecule (\(\ce{:B}\) in the medium in step#3, leading to a tetrahedral intermediate-II. Then the acidic medium protonates either \(\ce{-Nu}\) or \(\ce{-Lv}\)-groups. Protonation of \(\ce{-Nu}\) reverses the reaction but protonation of \(\ce{-Lv}\) makes it a better-leaving group in tetrahedral intermediate-III of step#4. The lone pair on \(\ce{O}\) re-establishes the \(\pi\)-bond with the electrophilic \(\ce{C}\), and at the same time, the leaving group leaves, leading to a protonated acyl product in the step#5. The protonated acyl donates its proton to any base \(\ce{:B}\) in the medium and becomes the product in step#6.
Acid is a catalyst in this mechanism as it is consumed in step#1 but re-generated in step#6, and accelerates two slow steps in this mechanism, i.e., step#2 and step#5. That is why it is called the acid-catalyzed nucleophilic substitution mechanism. Specifically, the protonation of the substrates \(\ce{R-\!\!{\overset{\overset{\huge\enspace\!{\overset{\Large{\delta{-}}}{O}}}|\!\!|\enspace}{\overset{\delta{+}}{C}}}\!\!-}\) converts it to a more reactive \(\ce{R-\!\!\!\!\!\!{\overset{\overset{\huge\enspace\enspace{\overset{\Large{+}}{O}H}}|\!\!\!\!\!\!\!|\enspace\enspace}{\overset{\delta{+}}{C}}}\!\!\!\!\!\!-\overset{\delta{-}}{Lv}}\) form that accelerates step#2, and protonation of \(\ce{-Lv}\) in tetrahedra intermediate-II converts it to a good leaving \(\ce{-Lv^{+}H}\) group in tetrahedral intermediate-III that accelerates step#5. The other steps in the mechanism are acid-base reactions which are inherently fast.
All the steps in this mechanism are reversible. It means there is an equilibrium between reactants and products. Three situations can arise, which are the following.
- If the incoming nucleophile (\(\ce{HNu}\)) is a poor nucleophile compared to the leaving group (\(\ce{HLv}\)), the reactants dominate,
- If the incoming nucleophile (\(\ce{HNu}\)) is a good nucleophile compared to the leaving group (\(\ce{HLv}\)), the products dominate, and
- If the nucleophilicity of the incoming nucleophile (\(\ce{HNu}\)) is comparable to that of the leaving group (\(\ce{HLv}\)), about an equal-equal mixture of reactants and products exists.
In situation#3, the equilibrium can be manipulated to favor products or reactants based on Le Chatelier’s principle. Employing one of the reactants in excess or removing one of the products drives the reaction forward. Similarly, adding one of the products in excess or removing one of the reactants drives the reaction in the reverse direction.
Neutral nucleophile and neutral substrate reaction mechanism
In this mechanism, both the nucleophile \(\ce{HNu}\) and the substrate \(\ce{R-\!\!{\overset{\overset{\huge\enspace\!{\overset{\Large{\delta{-}}}{O}}}|\!\!|\enspace}{\overset{\delta{+}}{C}}}\!\!-}\) are in their less reactive neutral forms in a neutral medium. Nucleophile \(\ce{HNu}\) attacks the electrophilic carbonyl \(\ce{\overset{\delta{+}}{C}}\) and, simultaneously, the \(\pi\)-bond breaks in step#1, leading to tetrahedral intermediate-I. The acidic proton on the incoming nucleophile in intermediate-I is removed by any base molecule (\(\ce{:B}\) in the medium in step#2, leading to tetrahedral intermediate-II, as shown below.

The nucleophilic \(\ce{O^{-}}\) in tetrahedral intermediate-II re-establishes the \(\pi\)-bond, and, simultaneously, either \(\ce{Nu^{-}}\) or \(\ce{Lv^{-}}\) leaves. Departure of \(\ce{Nu^{-}}\) reverses step#2 and departure of \(\ce{Lv^{-}}\) leads to the product in step#3. \(\ce{Lv^{-}}\) is neutralized by any acid molecule present in the medium in step#4.
Since both the nucleophile and the substrate are in their less reactive neutral forms, it works only in situations where a good leaving group (\(\ce{-Lv}\)) is attached to the acyl substrate. Step#3 is usually irreversible because the \(\ce{-Lv}\) groups chosen for these reactions are usually good leaving groups and poor nucleophiles.
Effect of leaving group on nucleophilic acyl substitution reactions
Good leaving groups (\(\ce{Lv^{-}}\)) are usually weak bases and poor nucleophiles at the same time. Weak bases do not fully share their electrons with acidic protons, and poor nucleophiles do not fully share their electrons with electrophilic \(\ce{C's}\). Poor nucleophiles increase the rates of slow steps in the following ways:
- their bond with \(\ce{C}\) is strongly polar, making the acyl \(\ce{\overset{\delta{+}}{C}}\) a more reactive electrophile, and
- they leave easily increasing the rate of collapse of tetrahedral intermediates.
Leaving groups commonly encountered in nucleophilic acyl substitution reactions are halogens like chlorine (\(\ce{-Cl}\)) or bromine (\(\ce{-Br}\)) , carboxylate (\(\ce{-O-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-R}\)), hydroxyl (\(\ce{-OH}\)), alkoxy (\(\ce{-OR}\)), and amine (\(\ce{-NH2}\)) groups. Their basicity and nucleophilicity increases in this order: \(\overrightarrow{\ce{Cl^{-} < R-COO^{-} < HO^{-} ≈ RO^{-} < ^{-}NH2}}\). Therefore, their ability to leave increases in the opposite order \(\overrightarrow{\ce{^{-}NH2 < HO^{-} ≈ RO^{-} < R-COO^{-} < Cl^{-}}}\), i.e., halogens like chlorine (\(\ce{-Cl}\)) are the best-leaving groups and amines (\(\ce{-NH2}\)) are the worst-leaving group.
These leaving groups are found in the following subclasses of acyl substrates: amides (\(\ce{R-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-NH2}\)), carboxylic acids (\(\ce{R-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-OH}\)), easters (\(\ce{R-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-OR}\)), acid anhydrides (\(\ce{R-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-O-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-R}\)), and acid halides (\(\ce{R-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-Cl}\)). These substrates are carboxylic acids and their derivatives. Their reactivity as acyl substrates in nucleophilic acyl substitution reactions increases in this order: \(\overrightarrow{\ce{R-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-NH2} < \ce{R-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-OH} ≈ \ce{R-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-OR} < \ce{R-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-O-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-R} < \ce{R-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-Cl }}\). It means a group that is higher in the reactivity order can be easily converted to a group that is lower in the order, but the reverse does not happen normally. For example, the following reaction is feasible:
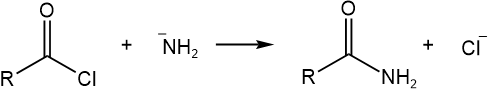 ,
,
but the following reaction, i.e., the reverse of it, does not happen:
 .
.
Examples of nucleophilic acyl substitution reactions
Reactions of acid halides
The acid halides, such as acid chlorides or bromides are the most reactive class of acyl substrates that can react with neutral nucleophiles and neutral substrates in a neutral medium. For example, acid chlorides can react with carboxylic acid, water, alcohol, or amines to form acid anhydrides, carboxylic acids, esters, and amines, respectively, as shown below.




Reactions of acid anhydrides
The acid anhydrides are reactive acyl substrates, second only to acid halides. Anhydride can react with water to produce two equivalents of carboxylic acid; with alcohols to produce one equivalent of ester and one equivalent of carboxylic acid; and with two amines to produce one equivalent of amide and one equivalent of an amine salt of carboxylic acid, as shown below.



Two moles of amine are needed in the last reaction because initially formed carboxylic acid reacts with the amine base producing an amine salt, as shown below.

Reactions of carboxylic acids
Carboxylic acids react with alcohols to produce ester but slowly. These reactions are reversible because the reactivity of the incoming nucleophiles is about the same as that of leaving nucleophoiles. Acid catalysis is applied to accelerate these reactions, as shown in the example below.

Since there is an equilibrium, excess alcohol is used, or water is removed to drive the reaction forward. These relations are called alcoholysis reactions because the reactant alcohol is also solvent. If a reverse reaction is desired, water is employed in excess as a solvent and the reaction is called hydrolysis of esters.
Carboxylic acids do not react with halides and another carboxylic acid because the incoming nucleophiles are poor relative to the leaving group. Base can not be added to accelerate the reactions of carboxylic acids because the base neutralizes the substrate, as shown below.

The carboxylate anion ( \(\ce{R-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-O^{-}}\) is the least reactive acyl substrate that is below amides in the reactivity order. Carboxylic acids do not react with amines for the same reason, i.e., amine base neutralizes the carboxylic acid producing ammonium salts, as shown below.

Protonated amines in the ammonium salts are not nucleophiles as they do not have lone pair of electrons on the \(\ce{N}\).
Reactions of esters
Esters are comparable in reactivity with carboxylic acids but without the acidic protons. Therefore, both acid-catalysis and base-promoted reactions can be carried out with esters. For example, water hydrolyzes esters under acidic conditions, as shown below.

It is a reversible reaction that can be driven forward by employing excess water or by removing the alcohol product. These reactions are called acid-catalyzed hydrolysis of esters.
The alkoxy group of esters can be substituted by an alkoxy group of another alcohol, as shown below.

It is a reversible reaction that can be driven forward by employing excess reactant alcohol or by removing the product alcohol. These reactions are called acid-catalyzed transesterification reactions.
Base-promoted reactions of esters
Transesterification of esters can be performed by employing a conjugated base of the alcohos, as shown in the following example.

Similarly, the example below shows that esters can be hydrolyzed using alkali, i.e., hydroxide ions.

The base-promoted hydrolysis of esters is called saponification which is used to hydrolyze fats, as shown in Figure \(\PageIndex{1}\). Fats are tri-esters of glycerol and fatty acids. Saponification of fasts produces sodium salts of fatty acids and glycerol. Sodium salts of fatty acids are soaps.
 Saponification reaction
Saponification reaction Examples of soaps
Examples of soapsReactions of amides
Amides are the least reactive among the acyl substrates. Amides do not react with halides, water, or alcohols under neutral conditions because the incoming nucleophiles are poor than the leaving group. However, water hydrolyzes amides under acid catalysis and heating, as shown below.

Acid converts poor leaving \(\ce{-NRR'}\) group to a good leaving \(\ce{-\overset{+}{N}HRR'}\) group, and also removes the product amine \(\ce{NRR'}\) by acid-base reaction, as illustrated below.

The ammonium ion (\(\ce{RR'N^{+}HCl^{-}}\) does not have a lone pair on \(\ce{N}\) and is not a nucleophile, that makes the reaction irreversible.
As shown below, alcohols react with amides under acid catalysis and heating.

Activating carboxylic acids in the laboratory and in biochemical systems
Carboxylic acids are common raw materials but must be converted into reactive derivatives, like acid halide, before conversion to other carboxylic acid derivates. Reagents like thionyl chloride (\(\ce{SOCl2}\)), phosphorous trichloride (\(\ce{PCl3}\)), or phosphorous pentachloride (\(\ce{PCl5}\)) are used to convert carboxylic acids into acid chlorides for this purpose, as shown below.



The above reagents are not available in biochemical systems. Adenosine triphosphate, shown below is a common reagent available in biochemical systems.
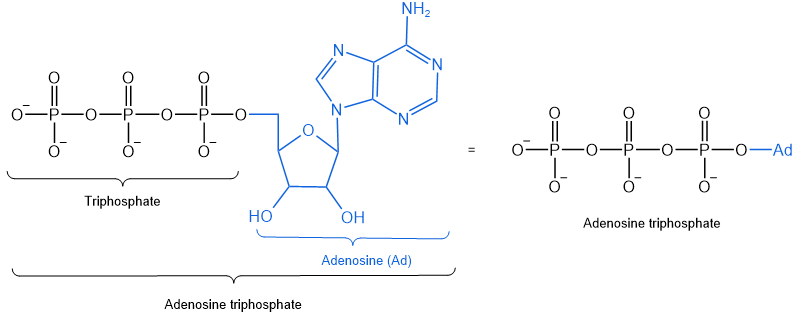
As illustrated below, adenosine triphosphate is used in biochemical systems to convert acylates into acyl phosphates or acyl adenylates.

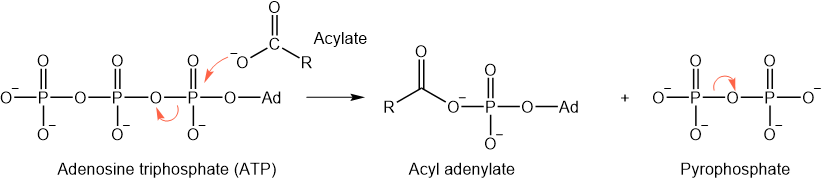
The phosphate and adenylate are good leaving groups. For example, coenzyme-A (\(\ce{CoASH}\)) containing nucleophilic thiol (\(\ce{-SH}\)) group is common in biochemical systems.

\(\ce{CoASH}\) displaces adenylate group from acyls through nucleophilic acyl substitution mechanism, as illustrated below.
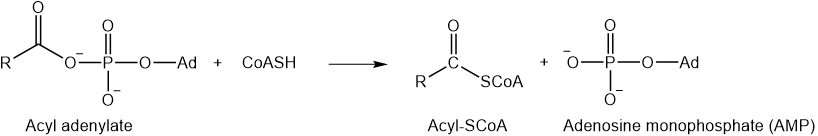
Thioesters, like \(\ce{-SCoA}\), are also good leaving groups. For example, the neurotransmitter acetylcholine is synthesized by reacting acetyl\(\ce{-SCoA}\) with choline through a nucleophilic acyl substitution mechanism, as shown below.

Condensation polymerization reactions
When two molecules combine to form a single molecule, usually with a loss of a small molecule like water or ammonia, the reaction is called a condensation reaction. For example, nucleophilic acyl substitution reactions between carboxylic acids and alcohols are condensation reactions, as shown below.

Polymers called polyesters are produced when molecules with two carboxylic acids and others with two alcohols condense to form esters. For example, benzene-1,4-dicarboxylic acid, commonly known as terephthalic acid, and ethane-1,2-diol, known as ethylene glycol, condense to produce a polyester called Polyethylene terephthalate (PET), as shown in Figure \(\PageIndex{2}\). Polyethylene terephthalate is used to make drink bottles and polyester fabrics.

Nucleophilic acyl substitution reactions between carboxylic acids and amines are another example of condensation reactions that can produce polyamides. For example, condensation of hexanedioic acid and hexane-1,6-diamine, known as hexamethylene diamine, produces a polyamide called nylon 66, as illustrated in Figure \(\PageIndex{3}\).
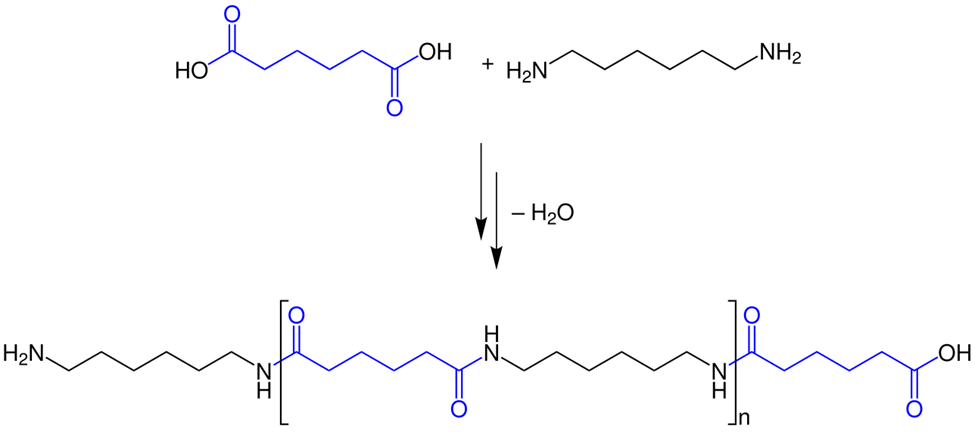
The first digit of 66 in the name nylon 66, tells number of carbons in one monomer (hexanedioic acid) and the second digit for the other monomer (hexane-1,6-diamine). Nylone 66 is commonly used in clothing, fishing lines, and guitar strings. Kevlar used to make bulletproof jackets is another example of polyamide, shown in Figure \(\PageIndex{4}\)

Condensation reaction between diisocyanates (\(\ce{O=C=N-R-N=C=O}\)) and alcohols (\(\ce{HO-R-OH}\)) produces polyurethane where ureththane groups ( \(\ce{-{\overset{\Large{H}}{N}}-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-O\bond{-}}\) ) link the monomer units in the polymer molecule, as illustrated in Figure \(\PageIndex{5}\).

Polyurethane makes foam, e.g., mattresses foam.

 Nucleic acids, i.e., DNA and RNA are biopolymers in which phosphate diester linkages hold the monomer units together, as illustrated in the figure on the right (copyright: Public domain). Nucleic acids are described in detail in a separate chapter. Proteins are polyamide bipolymers. For example, cinnamycin (lanthiopeptin), shown in the figure on the left (copyright: Public domain), is an antibacterial peptide produced by Streptomyces cinnamoneus containing 19 amino acids. Proteins are also described in detail in a separate chapter.
Nucleic acids, i.e., DNA and RNA are biopolymers in which phosphate diester linkages hold the monomer units together, as illustrated in the figure on the right (copyright: Public domain). Nucleic acids are described in detail in a separate chapter. Proteins are polyamide bipolymers. For example, cinnamycin (lanthiopeptin), shown in the figure on the left (copyright: Public domain), is an antibacterial peptide produced by Streptomyces cinnamoneus containing 19 amino acids. Proteins are also described in detail in a separate chapter.


