8.7: Making radioisotopes for medical uses
- Page ID
- 372955
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Natural radioisotopes usually have a long half-life and are not best suited for medical applications. The medical application usually requires short-lived radioisotopes. The radioisotopes are usually produced in nuclear reactors where particles, like \(\ce{\alpha}\)-particles, \(\ce{\beta}\)-particles, and neutrons, are abundant. Particle accelerators, such as the one shown in Fig. 8.7.1 also accelerate and direct the nuclear particles at the targets. The high-energy nuclear particles may be absorbed by and transmute the target nuclei to radioisotopes in a nuclear reaction.
Radioisotopes in medical applications are usually produced by the particle bombardment method. One reaction that happens naturally by neutron bombardment from cosmic rays on nitrogen-14 is the following.
\[\ce{_7^14N + _{0}^{1}{n} -> _6^14C + _{1}^{1}{p}}\nonumber\]
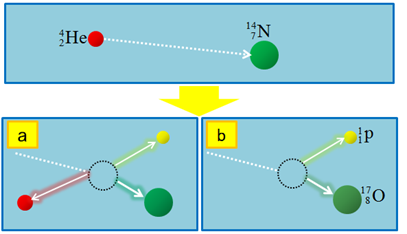
An example of an artificial nuclear reaction initiated by \(\ce{\alpha}\)-particle bombardment on nitrogen, observed by Rutherford that lead to the discovery of proton, is illustrated in Fig. 8.7.2.
\[\ce{_2^4He + _{7}^{14}{N} -> _8^17O + _{1}^{1}{p}}\nonumber\]
Another example is the nuclear reaction initiated by \(\ce{\alpha}\)-particles on beryllium, observed by James Chadwick, which lead to the discovery of the neutron.
\[\ce{_2^4He + _{4}^{9}{Be} -> _6^12C + _{0}^{1}{n}}\nonumber\]
An example of radioisotope production for medical uses is the following. Gold-198, used as a tracer in the liver, is produced by neutron bombardment on gold-197.
\[\ce{_79^197Au + _{0}^{1}{n} -> _79^198Au}\nonumber\]
Similarly, gallium-67 used in medical diagnostics is produced by proton bombardment on zinc-66.
\[\ce{_30^66Zn + _{1}^{1}{p} -> _31^67Ga}\nonumber\]
Molybdenum-99 is a radioactive isotope produced in a nuclear reactor by neutron bombardment of molybdenum-98.
\[\ce{_42^98Mo + _{0}^{1}{n} -> _42^99Mo} + \gamma\nonumber\]
Molybdenum-99 is also produced as a fission product of uranium-235. Molybdenum-99 decays to technetium-99m that has several uses in nuclear medical imaging and treatment.
\[\ce{_42^99Mo -> _43^{99m}{Tc} + _{-1}^{0}{e}}\nonumber\]

Technetium-99m is short-lived (half-life 6 h), and needs to be produced in the hospital to minimize its decay during the transport. Its parent molybdenum-99 has a half-life of 66h and can be transported without significant decay during the transport. Molybdenum-99/technetium-99m generators are supplied to the hospitals in a shielded container. Fig. 8.7.3 illustrates the first Molybdenum-99/technetium-99m generator developed at Brookhaven National Laboratory. Molybdate (MoO42) ion is adsorbed onto alumina adsorbent in a column. When molybdenum-99 decays to technetium-99m, the ion change to pertechnetate (TcO4‑), which is less tightly bound to the alumina. Pouring a saline solution through the column elutes the technetium-99m as TcO4‑ ion, which is then used for medical purposes in the hospitals.
Destruction of an inoperable tumor has also been tested by \(\ce{\alpha}\)-emission from boron-10 upon neutron bombardment.
\[\ce{_{0}^{1}{n} + _5^10B -> _4^7Li + _2^4He}\nonumber\]


