8.6: Medical uses of radioisotopes
- Page ID
- 372954
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The use of nuclear chemistry in medical technologies is increasing over time. The medical uses can be divided into two categories :
- medical imaging of organs or diagnosing any malfunction and
- therapeutic use, mainly for killing cancerous cells.
Radioisotopes in medical imaging
- those that emit \(\ce{\gamma}\)-rays, directly or indirectly, during their decay,
- can be delivered to the organ of interest in pure form or tagged in a compound,
- are short-lived or can be excreted from the body after use.
Table 8.6.1 lists some radioisotopes commonly used in medical imaging. A low dose of the radioisotope is administered to a patient. The \(\ce{\gamma}\)-rays cross over the body and are recorded like X-rays. A computer finally converts the recording into a useful image. The image is compared with an image of a healthy organ to diagnose any malfunction.
| Radioisotope | Symbol | Mode of decay | Half-life | Use in medical imaging |
|---|---|---|---|---|
| Carbon-11 | \(\ce{_6^11C}\) | β+, g | 20.3 m | Brain scan to trace glucose |
| Fluorine-19 | \(\ce{_9^18F}\) | β+, g | 109m | Brain scan to trace glucose |
| Chromium-51 | \(\ce{_24^51Cr}\) | E.C., g | 27.3 d | Diagnose albinism; image the spleen and gastrointestinal track |
| Gallium-67 | \(\ce{_31^67Ga}\) | E.C., g | 78.3 h | Whole-body scan for tumors |
| Selenium-75 | \(\ce{_34^75Se}\) | E.C., g | 118 d | Pancreas scan |
| Krypton-81m | \(\ce{_36^{81m}Kr}\) | g | 13.3 s | Lung ventilation scan |
| Xenon-133 | \(\ce{_54^133Xe}\) | β | 5.24 d | Lung ventilation scan |
| Strontium-81 | \(\ce{_38^81Sr}\) | β | 22.2 m | Scan for bone cancer and bone diseases |
| Mercury-197 | \(\ce{_80^197Hg}\) | E.C., g | 64.1 h | Kidney scan |
| Iron-59 | \(\ce{_26^59Fe}\) | β, g | 44.5 d | Bone marrow function and anemias |
| Iodine-131 | \(\ce{_53^131I}\) | β, g | 8.04 d | Diagnosis of thyroid malfunction |
| Iodine-123 | \(\ce{_53^123I}\) | E.C., g | 13.2 h | Diagnosis of thyroid malfunction |
| Technetium-99m | \(\ce{_43^{99m}Te}\) | g | 6.01 h | Brain, liver, kidney, bone scans, and diagnosis of the damaged heart muscle |
| Phosphorous-32 | \(\ce{_15^32P}\) | β | 14.3 d | Detect eye tumors |
| Thallium-201 | \(\ce{_81^201Tl}\) | E.C., g | 3.05 d | Heart scan and exercise stress test |
An example of medical imaging is the thyroid gland in the neck that produces the hormone thyroxin, which controls the overall rate of metabolism in the body. Each thyroxin molecule contains four iodine atoms. Administration of radioactive Na131I or Na123I salt accumulates the iodine in the thyroid gland in a few hours. Decay of 131I and 123I involves \(\ce{\gamma}\)-emission.
\[\ce{_53^131I -> _54^131Xe + _{-1}^{0}{e}} + \gamma\nonumber\]
\[\ce{_53^123I + _{-1}^{0}{e} -> _52^123Xe} + \gamma\nonumber\]
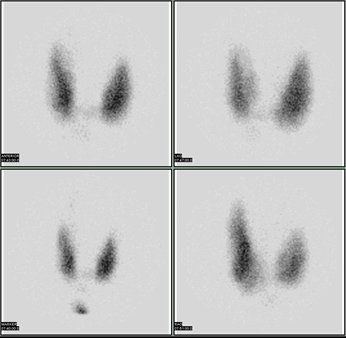
The \(\ce{\gamma}\)-emission from the iodine localized in the thyroid gland is recorded, as shown in Fig. 8.6.1. An overactive thyroid (hyperthyroidism) cumulates more and underactive thyroid (hypothyroidism) cumulates less iodine than a healthy thyroid.
Another example is positron emission tomography (PET). Positron emitters like carbon-11 and fluorine-18 incorporated in a suitable compound like glucose allow following the metabolic path of the compound. For example, 18-fluorodeoxyglucose )18-FDG) is a glucose molecule in which one of the oxygen is replaced with 18F. Intravenous injection of the 18-FDG ultimately results in the cumulation of 18-FDG in the brain and other body organs where glucose is used in the metabolic process. The 18F emits a positron, which, being an anti-particle of the electron, reacts with the electron and produces two g \(\ce{\gamma}\)-rays.
\[\ce{_9^18F -> _8^18O + _{1}^{0}{e}} + \gamma\nonumber\]
\[\ce{_{1}^{0}{e} + _{1}^{0}{e} ->} 2\gamma\nonumber\]
The \(\ce{\gamma}\)-rays are used to obtain an image of the organ. The image reveals problem areas in the form of an abnormal concentration of glucose in the part of the organ. For example, Fig. 8.6.2 compares the PET image of a healthy brain versus a brain with Alzheimer's disease.


Since glucose metabolism happens in all organs, whole-body PET scans can be used to diagnose lung, colorectal, head and neck, and esophageal cancers as well as other diseases that involve abnormal glucose metabolism. For example, tumors have high metabolic rates; the PET scans using 18-FDG are used to detect them, as shown in Fig. 8.6.3.

Medical imaging without using radioisotopes
Major examples of medical imaging using external radiation sources include the following.
- X-rays, which are external radiation source, is commonly used for medical imaging of organs and bones.
- Computed tomography (CT) scan uses computer processing of many X-ray measurements, from different angles, to produce a cross-sectional view (virtual slices) of the organ.
- Magnetic resonance image (MRI) is another powerful medical imaging technique that is based on the fact that hydrogen atoms splint into two two energy states when placed in a strong magnetic field. When illuminated with infrared (IR) radiation of the energy matching with the energy gap between the two groups, the hydrogen atoms are excited from the lower to higher energy state. The decay of the excited state emits the IR radiations that are recorded to obtain the image of soft tissues that contain many hydrogen atoms in the form of water molecules. Fig. 8.6.4 shows some examples.

Radiation therapy
The purpose of radiation therapy is to selective kill the diseased cells or tissues by exposing them to radiation. Higher radiation doses are required for therapy than for imaging. The radiation source can be external or internal.
External radiation therapy
In the external irradiation, the radiation from the source, such as coba which are often used, other radiation sources are being developed, such as proton beam from the cyclotron, which have been used to kill inoperable tumors near or in the eye, skull base, or spine. Proton therapy uses a beam of proton to deliver radiation directly to the tumor.

Internal radiation therapy
Internal radiation therapy is used when a short-lived radioisotope can be made to selectively concentrate in the organ or tissue of interest. For example, iodine-131 is \(\ce{\beta}\) and \(\ce{\gamma}\)-emitter, is administered to a patient, is picked up by the thyroid gland, and is used to treat hyperthyroidism. Another example is actinium-225, which is an \(\ce{\alpha}\)-emitter with a half-life of 10 days. Actinium-225 installs in a monoclonal antibody that is attached to a prostate-specific antigen and delivered selectively to treat prostate tumors.

Brachytherapy
Brachytherapy or seed implantation is a form of internal radiation treatment that delivers a high dose for a short period compared to the external radiation treatment. Fig. 8.6.6 shows the sites in the body where brachytherapy can be used to treat cancer. For example, 40 or more titanium capsules, about the size of a rice grain (Fig. 8.6.7), are implanted to treat prostate cancer. The seed contains \(\ce{\gamma}\)-emitter like iodine-125 (half-life 60 days), palladium-103 (half-life 17 days), or cesium-131 (half-life 10 days). The seed may be left in the body because, due to the short half-life, they are no more significantly radioactive after the treatment.

Another option is temporary brachytherapy, e.g., iridium-192 needles that deliver higher radiation doses are inserted to treat prostate cancer and removed after 5 to 10 min. The iridium-192 wires are also used as a follow-up treatment after breast cancer surgery to kill any residual or recurring cancer cells. The iridium-192 wires are inserted through a catheter implanted in the space from where the tumor was removed. The wires are removed after delivering the required radiation dose. The process is repeated twice a day for five days. The catheter is removed, and no radioactive material is left in the body after the treatment.


