8.5: Ionizing radiation exposures
- Page ID
- 372953
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Interaction of radiation with matter
The ionizing radiations knock off electrons from atoms and molecules. Water composes about 60% of the human body. The oxygen atom in water has eight valence electrons, four in the two bonding pairs, and four in the two lone pairs, as shown in Fig. 8.5.1. If one of them is knocked off by radiation, the result is a radical-cation. It is radical because it has one unpair electron and its octet is incomplete, and cation because an electron has been lost. The radical cation can then release a proton to become a hydroxyl radical. The radicals are very reactive species due to the incomplete octet. The radicals tend to react with any material around that causes damage to the tissues. Notably, the damage to DNA is the most dangerous, causing mutation, cancer, and hereditary problems.

The cells that are proliferating are more susceptible to the harmful effects of radiation exposure, including bone marrow, skin, reproductive organs, and intestinal lining, as well as all the cells of growing children. If the bone marrow cells are damaged, the red blood cells may not be produced. Damage to the reproductive cells or the cells of a fetus may cause congenital disabilities. Fortunately, the cancer cells are multiplying and affected by the radiation exposure much more than the surrounding healthy cells, which allows for selectively killing the cancerous cells by radiation treatment. The use of ionizing radiations in cancer treatment is described in a later section.
Effects of radiation exposure on humans
Exposure of humans to less than 0.25 Sv usually does not have any noticeable effect. Whole-body exposure of 1 Sv results in a temporary decrease in the white blood cell counts. More than 1 Sv exposure may cause nausea, vomiting, fatigue, and a reduction in white blood cell count. More than 3 Sv doses to the whole body can decrease the white blood cell count to zero and cause diarrhea, hair loss, and infection. Exposure to 5 Sv can cause death in 50% of the people receiving the dose –it is called a lethal dose for one-half of the population (LD50). Whole-body exposure of 6 Sv or higher is fatal to all humans within a few weeks. Fig. 8.5.2 illustrates the effects.
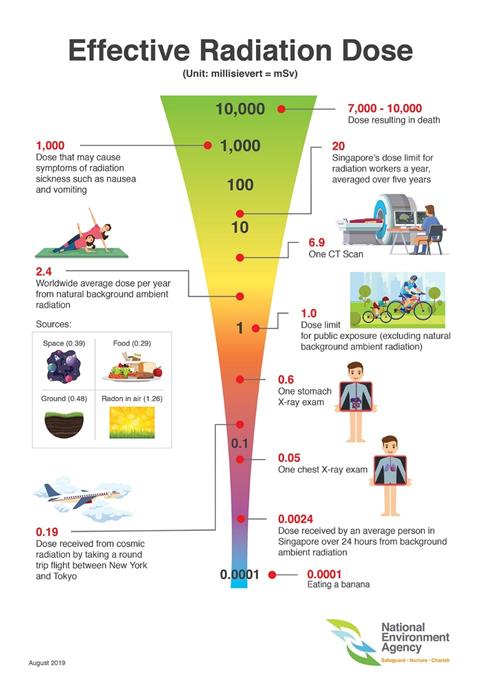
Background radiation exposures
Humans are exposed to radiation in the cases of nuclear accidents, during nuclear radiation treatments, particularly for treating different forms of cancers, and during medical imaging for medical diagnostic purposes. Besides these human-made radiation exposures, humans are regularly exposed to natural sources of radiation, called background radiation. The background radiation can be in foods, e.g., potassium-40 is a naturally occurring radioactive isotope present in potassium-containing foods. Carbon-14, radon-222, strontium-90, and iodine-131 are other radioisotopes present in the air and foods around us. Radioactive isotopes of uranium and thorium and their decay products are the source of radiation in soil. The extraterrestrial sources of radiation are cosmic rays that are stopped in the upper atmosphere, but some may reach the ground and expose the people. The average annual radiation dose per person in the U.S. is 6.2 millisieverts (620 millirem).

Radiation protection
Different types of radiation have different penetration depths in the air and the human body. The \(\ce{\alpha}\)-particles are heavy with two protons and two neutrons; they cause much ionization but travel a few centimeters in air. A sheet of paper can stop the \(\ce{\alpha}\)-particles, as illustrated in Fig. 8.5.3. Exposure to \(\ce{\alpha}\)-particles from an external source affects only the outer layer of the skin. However, an internal source can cause significant damage to the nearby tissues, as illustrated in Fig. 8.5.4
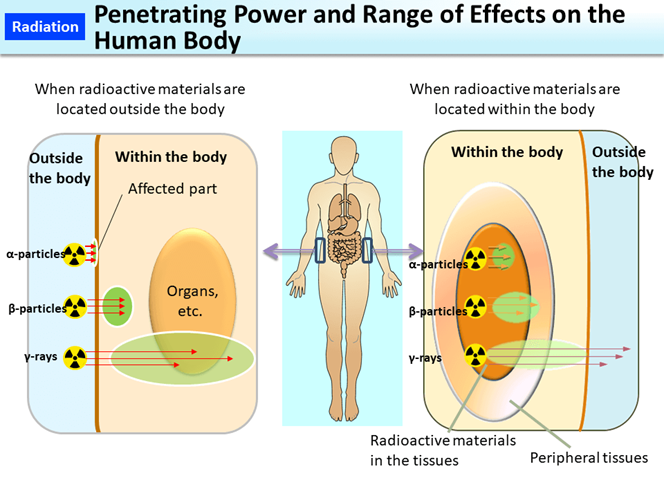
The \(\ce{\beta}\)-particles are fast-moving electrons that can travel several meters in the air. A 2-4 mm thick aluminum plate can stop them. The \(\ce{\beta}\)-particles can penetrate 4 to 5 centimeters into the tissue. External exposure to the \(\ce{\beta}\)-particles can burn the skin, but internal organs remain safe. Internal exposure to the \(\ce{\beta}\)-particle is more dangerous than external exposure.
The \(\ce{\gamma}\)-rays and X-rays can travel long distances in air, up to half a kilometer, and are not easily stopped by common material. A thick layer of lead or concrete shield is required to stop the \(\ce{\gamma}\)-rays and X-rays. External exposure to the \(\ce{\gamma}\)-rays and X-rays is the most dangerous because these rays can penetrate deep and damage the organs.
Neutrons are high-energy neutral particles that also have high penetrating power. They lose energy by colliding with atoms of substances. The most effective shielding is the water or concrete that has moisture in it to stop the neutrons effectively.
The workers in a radiation environment wear heavy clothes, gloves, and lab coats to provide additional protection. The radioactive materials are usually stored in shielded containers, even the syringes containing radioactive materials for injection are shielded. The general rules for protection against the radiation are:
- keep minimum possible time in the radiation environment -less the time means less exposure,
- keep as much distance from the radiation source as possible -the radiation intensity drops inversely proportional to the square of the distance, and
- keep shielding between you and the source of radiation as much as possible -the more the shielding the less the exposure.


