6.8: pH Buffers
- Page ID
- 372205
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)What is a pH buffer?
A pH buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the pH change to be minimal when a small amount of a strong acid or a strong base adds to it.
For example, the addition of 0.020 mol HCl into 1 L of water changes pH from 7 to 1.7, i.e., about an 80% change in pH. The addition of 0.020 mol NaOH to the same water changes pH from 7 to 12.3, i.e., again, about an 80% change in pH. In contrast to pure water, 1 L of buffer solution containing 0.50 mol acetic acid (CH3COOH) and 0.50 mol CH3COO- -the conjugate of the acetic acid, changes pH from 4.74 to 4.70 by the addition of the same 0.020 mol HCl and from 4.74 to 4.77 by the addition of 0.020 mol NaOH, i.e., about 1% change in pH, as illustrated in Fig. 6.8.1.
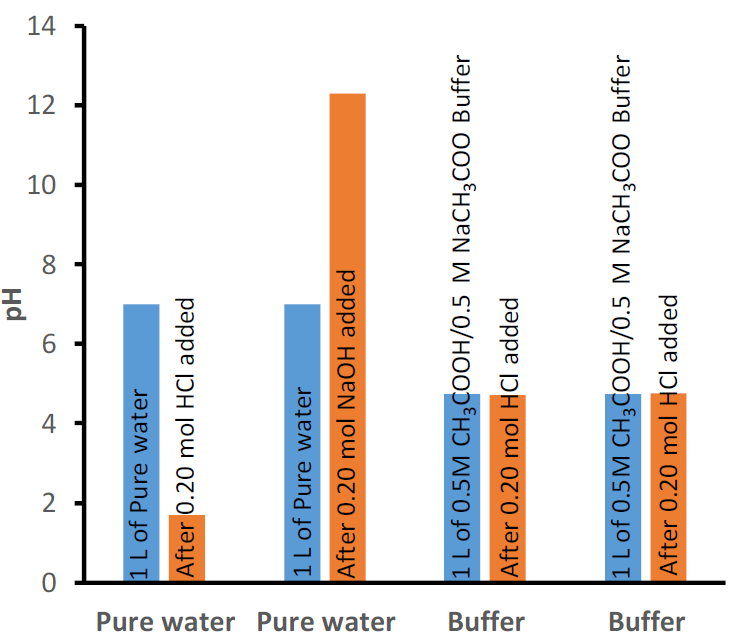
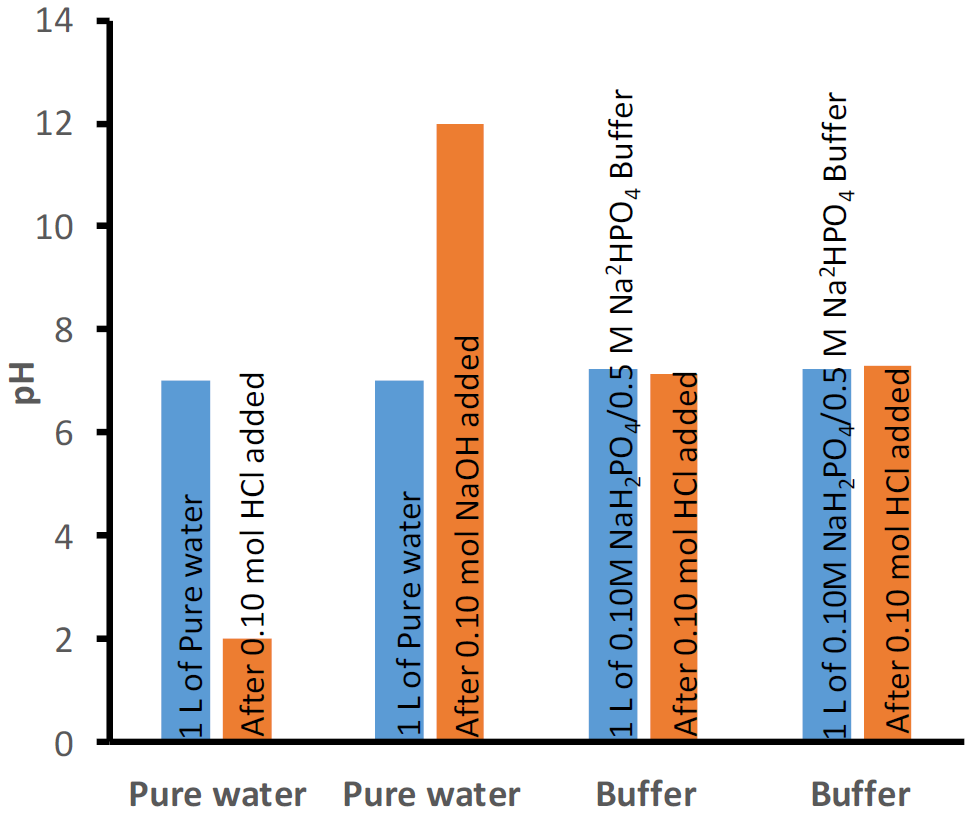
Buffers of different initial pH values can be prepared, e.g., by varying the ratio of the weak acid to its conjugate base or by using a different set of a weak acid and its conjugate base. One example is a buffer of initial pH 4.74 comprising 0.5 M acetic acid and 0.5 M sodium acetate shown in Fig. 6.8.1. Another example is a buffer comprising 0.1M dihydrogen phosphate and 0.1M hydrogen phosphate that has an initial pH of 7.21, as shown in Fig. 6.8.2.
Buffer capacity refers to how much amount of a strong acid or a strong base the buffer can handle before a drastic change in pH happens.
Higher the amount of weak acid/conjugate base higher the buffer capacity. The buffer has an equal amount of weak acid and its conjugate base has a higher buffer capacity than the same buffer that has an unequal ratio of the acid and its conjugate base.
Strong acid and its conjugate base or a strong base and its conjugate acid do not make a buffer solution.
Mechanism of buffer action
The buffer contains a weak acid and its conjugate base in equilibrium. For example, acetic acid/sodium acetate buffer has the following equilibrium.
\begin{equation}
\mathrm{CH}_{3} \mathrm{COOH} \rightleftarrows \mathrm{H}^{+}+\mathrm{CH}_{3} \mathrm{COO}^{-}\nonumber
\end{equation}
The molar concentration of hydrogen ions [H+] defines the pH of the solution. The conjugate base consumes any strong acid added.
\begin{equation}
\mathrm{HA}+\mathrm{CH}_{3} \mathrm{COO}^{-} \rightarrow \mathrm{CH}_{3} \mathrm{COOH}+\mathrm{A}^{-}\nonumber
\end{equation}
, where HA is any strong acid and A- is its conjugate base. The concentration of CH3COOH increases and CH3COO- decrease, but pH decreases small because [H+] increases only a little. Similarly, the weak acid consumes any strong base added.
\begin{equation}
\mathrm{MOH}+\mathrm{CH}_{3} \mathrm{COOH} \rightarrow \mathrm{CH}_{3} \mathrm{COO}^{-}+\mathrm{M}^{+}+\mathrm{H}_{2} \mathrm{O}\nonumber
\end{equation}
Where MOH is any strong base, and M+ is its conjugate acid. Fig. 6.8.3 illustrates the mechanism of buffer action.
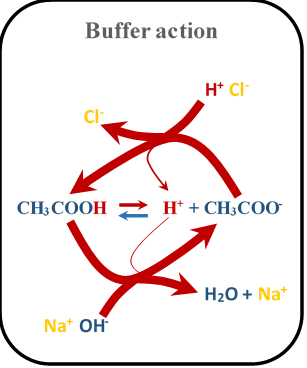
Strong acid and its conjugate base mixture have no buffer action because the acid dissociates almost 100%, leaving no acid behind for the buffer action. The conjugate base of a strong acid is a very weak base that does not react with the added acids. The same explanation applies to a strong base and its conjugate acid mixture having no buffer action.
Buffers in the blood
The blood maintains its pH of ~7.4 primarily by the carbonic acid/hydrogen carbonate buffer system. The blood pH in the range of 7.45 to 7.35 is considered healthy, but outside of this range causes medical problems. If the blood pH decreases to 6.8 or increases to 8.0, death may occur. It is critical to maintain the blood pH in a narrow range for the cells to function correctly. Specifically, the enzymes and other proteins have secondary, tertiary, and quaternary structures needed for their proper functions. Hydrogen bonding plays a crucial role in defining the protein’s structure. pH changes alter the hydrogen bonding making the proteins less effective or ineffective in their functions.
Buffer systems regulate the blood pH. The primary blood buffer system is carbonic acid/hydrogen carbonate, as illustrated in Fig. 6.8.4. Metabolic processes in the cells produce carbon dioxide (CO2) that enters the bloodstream and produces carbonic acid (H2CO3) by reacting with water. Kidneys supply the hydrogen carbonate (HCO3-) –the conjugate base of the carbonic acid and also keep a reservoir of the HCO3-. The H2CO3 consumes any base added and the HCO3- consumes any acid added, thus minimizing the pH change due to the added acids and bases.
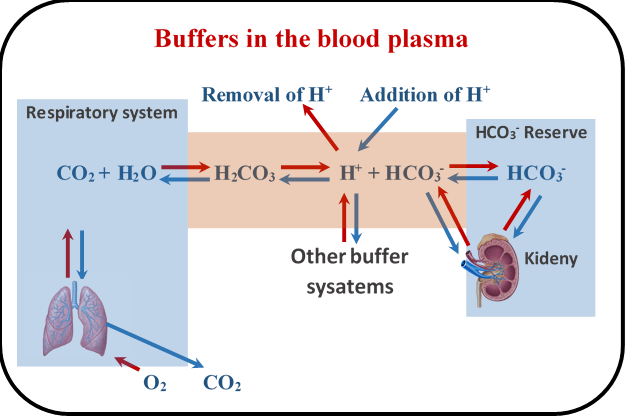
The primary mechanism for pH regulation by the H2CO3/HCO3- buffer in the blood is through the lungs. When the blood pH is acidic compared to the average, the breathing rate increases exhaling more CO2 that decreases the concentration of H+, following the blue arrows in Fig. 6.8.4, increasing the pH. The decrease in the breathing rate has the opposite effect. Kidneys also regulate the blood pH by adding or removing HCO3-, but the kidney’s response is delayed compared to the response of the lungs.


