5.5: Osmosis
- Page ID
- 372212
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Semipermeable membranes surround living cells and organelles in the cells. The semipermeable membranes allow water and small molecules but do not allow the passage of large molecules and ions.
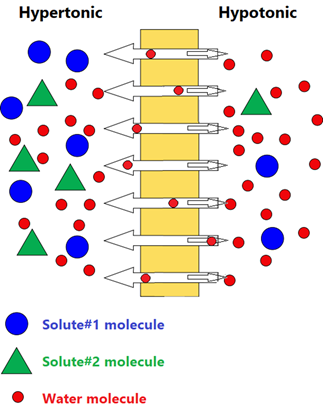
Osmosis is the passage of water and small molecules across a semipermeable membrane with a net flow from a less concentrated solution to a more concentrated solution.
Osmosis helps in the absorption, retention, and flow of water, nutrients, and other molecules required in the biological systems. Fig. 5.5.1 illustrates the process of osmosis.
Osmotic pressure
Osmosis causes net water flow from the less concentrated to the more concentrated solution across the semipermeable membrane. Consequently, the water level rises in the less concentrated solution compartment, as illustrated in Fig. 5.5.2. The difference in the height of water increases and applies pressure, pumping water back to the more concentrated side until the flow of water is equal on the two sides.
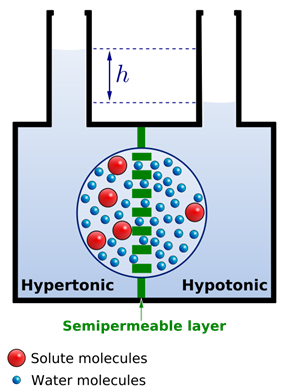
The pressure that prevents additional water flow to the more concentrated solution side of the semipermeable membrane is called osmotic pressure.
The osmotic pressure is proportional to the overall concentration of the solute particles. For example, 0.1 molar NaCl has osmotic pressure about twice that of 0.1 molar glucose because each mole of glucose adds one mole of solute particles, while each mole of NaCl produces two moles of particles, i.e., one-mole Na+ and one-mole Cl- ions in the solution.
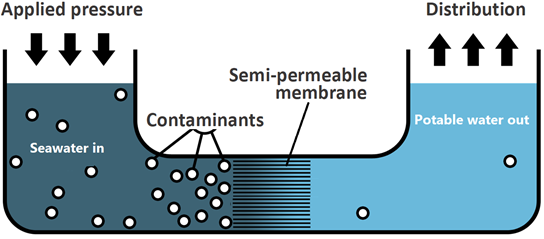
Reverse osmosis is the net flow of water to the less concentrated or pure water across the semipermeable membrane by applying external pressure more than the osmotic pressure on the more concentrated solution side.
Reverse osmosis is the net flow of water to the less concentrated or pure water across the semipermeable membrane by applying external pressure more than the osmotic pressure. Note that solvent flow in reverse osmosis driven by external pressure is the opposite of regular osmosis. Reverse osmosis is used to produce drinking water from seawater sources, as illustrated in Fig. 5.5.3.
Isotonic, hypertonic, and hypotonic solutions
Cell membranes are semipermeable membranes that separate intracellular and extracellular fluids. The concentration difference across the membrane and the resulting osmotic pressure plays an essential role in cell functions. Intravenous solutions injected into patients must have the same osmotic pressure as the body fluids.
Solutions with the same solute particle concentration and osmotic pressure are called isotonic. If the two solutions across a semipermeable membrane do not have the same solute particle concentration, the solution with higher solute particle concentration and higher osmotic pressure is hypertonic, and the other has lower solute particle concentration and lower osmotic pressure is hypotonic.
Remember that hyper- means more and hypo-means less, concerning solute particle concentration in the case of osmosis.
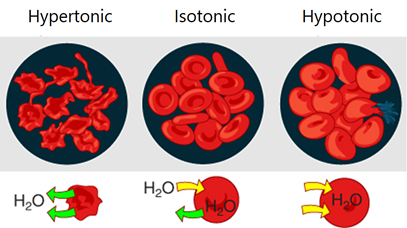
Cells placed in an external solution may retain their size, shrink, or swell depending on the relative osmotic pressure of fluid inside and outside of the cell, as illustrated in Fig. 5.5.4. For example, red blood cells placed in an isotonic solution retain their size because the flow of water into and out of the cell is the same.
- Typical isotonic solutions are 0.9% m/v NaCl solution in water or 5% m/v glucose solution in water.
- Red blood cells placed in a hypertonic solution shrink in size due to more flow of water out than into the cell –a process called crenation.
- Red blood cells placed in a hypotonic solution swell and burst due to more water flow into than out of the cells –a process called hemolysis.
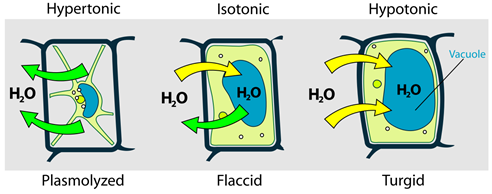
A similar situation happens in plant cells that are placed in different environments concerning osmotic pressure, as illustrated In Fig. 5.5.5.
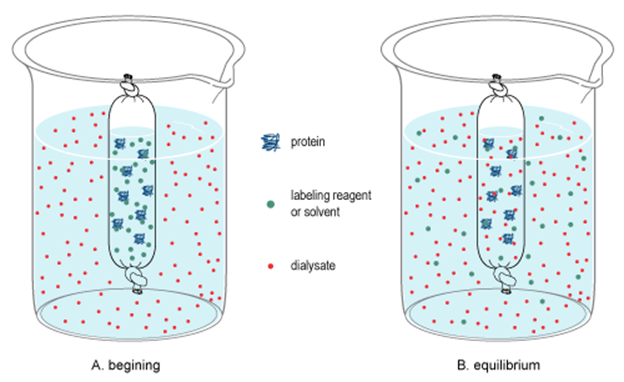
Dialysis
Dialysis separates colloids from water, dissolved ions, and molecules of small dimention
Dialysis is similar to osmosis with the difference that in dialysis water, small molecules and ions can pass through a dialyzing membrane leaving behind collide particles like proteins and starch molecules, as illustrated in Fig. 5.5.6.
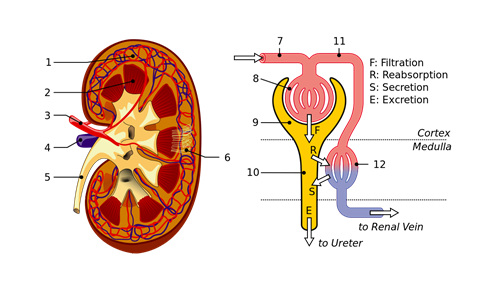
Role of dialysis in the human body
Kidneys filter the blood by dialysis process. There are over a million tubular structures, called nephrons, in the kidney surrounded by dialyzing membranes. The nephrons filter the water, small molecules like glucose, amino acids, urea, and ions from the blood. Useful products and most of the water reabsorb later on, but urea and other waste products excrete through urine, as illustrated in Fig. 5.5.7.
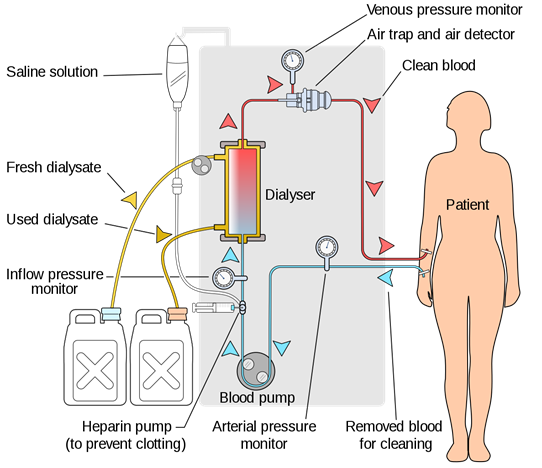
Hemodialysis
Hemodialysis is used to extract urea and other waste products from the blood when a person's kidney fails to remove them, as illustrated in Fig. 5.5.8. A hemodialysis system is a kind of artificial kidney in which the blood flows through long cellophane tubes placed in an isotonic solution containing NaCl, KCl, NaHCO3, and glucose. Cellophane is a dialyzing membrane that does not let proteins, other large molecules, and blood pass through it, but urea excretes.