5.4: Concentration of solutions
- Page ID
- 372211
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The concentration of a solution tells the amount of solute dissolved in a given amount of solution.
Making a solution of know concentration
A measured amount of solute is dissolved in enough solvent to make the desired volume of the solution, as illustrated in Fig. 5.4.1. The concentration of the solution can be expressed in different ways using mass, volume, or mole units, as explained in the following.
Concentration in percent (%)
Percentage (%) is a number or ratio that represent a fraction of 100.
For example, 5% means 5:100, where 5 is the part, and 100 is the total. The percentage is calculated as a hundred times of part by total, i.e.,
\begin{equation}
\text { Percentage }(\%)=\frac{\text { part }}{\text { Total }} \times 100 .\nonumber
\end{equation}
A 50.0 g NaCl is dissolved in water to make a 500 g solution. What is the percentage of NaCl in the solution?
Solution
Given part = 50.0 g NaCl, and total = 500 g solution. Desired: %NaCl in the solution?
Formula: \(\text { Percentage }(\%)=\frac{\text { part }}{\text { Total }} \times 100 \text {. }\nonumber\)
Calculations: \(\text { Percentage }(\%)=\frac{50.0 cancel{\mathrm{~g}}}{500 cancel{\mathrm{~g}}} \times 100=10.0 ~\% \mathrm{NaCl}\)
The units cancel in the fraction calculation part, and a % sign is added to the answer to tell that it is a fraction out of a hundred.
Mass percent (m/m)% concentration
The mass percent concentration expresses the mass units of solute in a hundred mass units of the solution.
\begin{equation}
\operatorname{Mass}(\%)=\frac{\text { mass of solute }(g)}{\text { mass of solute }(g)+\text { mass of solvent }(g)} \times 100=\frac{\text { mass of solute }(g)}{\text { mass of solution }(g)} \times 100\nonumber
\end{equation}
Note that the total is solute and solvent added together, i.e., solution.
What is the mass % of NaOH in a solution prepared by dissolving 10.0 g NaOH in 100 g water?
Solution
Given solute = 10.0 g, and solvent = 100 g, Desired: Mass% NaOH?
Formula: \(\text { Mass }(\%)=\frac{\text { mass of solute }(g)}{\text { mass of solute }(g)+\text { mass of solvent }(g)} \times 100\)
Calculations: \(\text { Mass }(\%)=\frac{10.0 \mathrm{~g} \mathrm{NaOH}}{(10.0 \mathrm{~g}+100 \mathrm{~g}) \text { solution }} \times 100=9.09 \% \mathrm{NaOH}\)
Note that the mass% concentration and its reciprocal are two conversion factor: \(\frac{\text { given } g \text { solute }}{100 g \text { solution }}\) and \(\frac{100 g \text { solution }}{\text { given g solute }}\)
Neosporin antibiotic is a 3.5% m/m neomycin solution. How many grams of neomycin are in 50 g of ointment?
Solution
Given: \(3.5 \% \text { neomycin }=\frac{3.5 \mathrm{~g} \text { neomycin }}{100 \mathrm{~g} \text { solution }}\), and Solution amount = 50 g, Desired: ? g neomycin
Calculations: \[50 \cancel{\mathrm{~g} \text { solution }} \times \frac{3.5 \mathrm{~g} \text { neomycin }}{100 \cancel{\mathrm{~g} \text { solution }}}=1.8 \mathrm{~g} \text { neomycin. }\nonumber\]
Volume percent (v/v)% concentration
The volume percent concentration expresses the volume units of solute in a hundred volume units of the solution.
The mathematical form of the v/v % is:
\begin{equation}
\text { Volume }(\%)=\frac{\text { volume of solute }(\mathrm{mL})}{\text { volume of solution }(\mathrm{mL})} \times 100\nonumber
\end{equation}
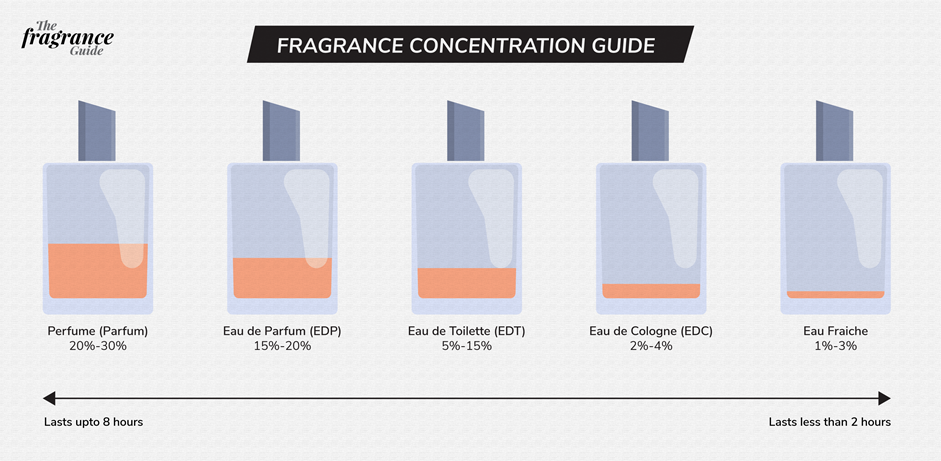
Fig. 5.4.2 shows the volume percent concentration ranges of different classes of fragrances.
What is the volume % of rose extract in a solution prepared by dissolving 14.0 mL rose extract in a solvent to make 200 mL of solution?
Solution
Given: Solute = 14.0 g, and solution = 200 g, Desired: Volume% rose solution?
Formula: \begin{equation}
\text { Volume }(\%)=\frac{\text { volume of solute }(m L)}{\text { volume of solution }(m L)} \times 100\nonumber
\end{equation}
Calculations: \begin{equation}
\text { Volume }(\%)=\frac{14 \mathrm{~mL} \text { rose extract }}{200 \mathrm{~mL} \text { solution }} \times 100=7.0 \% \text { rose solution }\nonumber
\end{equation}
The two conversion factors for v/v % concentration are: \begin{equation}
\frac{\text { given mL solute }}{100 \mathrm{~mL} \text { solution }} \quad\text {, and }\quad \frac{100 \mathrm{~mL} \text { solution }}{\text { given } \mathrm{~mL} \text { solute }}\nonumber
\end{equation}
What is the volume of bromine (Br2) in 250 mL of 4.8% v/v of Br2 solution in carbon tetrachloride?
Solution
Given: Concentration 4.8% v/v bromine \(=\frac{4.8 ~m L \text { bromine }}{100 ~m L \text { solution }}\), volume of solution = 250 mL, Desired: Volume of solute, i.e., ? mL bromine.
Calculations: \[
250 \mathrm{~mL} \text { solution } \times \frac{4.8 \mathrm{~mL} \text { bromine }}{100 \mathrm{~mL} \text { solution} .}=12 \mathrm{~mL ~bromine} \text {. }\nonumber\]
Mass/volume percent (m/v)% concentration
The mass/volume percent concentration expresses the mass units of solute in a hundred volume units of solution.
Mathematical form of m/v % is:
\begin{equation}
\text { Mass/volume }(\%)=\frac{\text { mass of solute }(g)}{\text { volume of solution }(m L)} \times 100\nonumber
\end{equation}
What is the mass/volume % of glucose solution prepared by dissolving 50 g glucose in enough water to make 1000 mL of solution?
Solution
Given: Solute = 50.0 g, and Solution = 1000 mL, Desired: Mass/volume % glucose solution?
Formula: \begin{equation}
\text { Mass/volume }(\%)=\frac{\text { mass of solute }(g)}{\text { volume of solution }(m L)} \times 100\nonumber
\end{equation}
Calculations: \begin{equation}
\frac{\text { Mass }}{\text { volume }}(\%)=\frac{50 \mathrm{~g} \text { glucose }}{1000 \mathrm{~mL} \text { solution }} \times 100=5.0 \% \text { glucose solution by } \frac{\mathrm{m}}{\mathrm{v}} .\nonumber
\end{equation}
The two conversion factors from ms/v % concentration are: \begin{equation}
\frac{\text { given } g \text { solute }}{100 \mathrm{~mL} \text { solution }}\quad \text { and }\quad \frac{100 \mathrm{~mL} \text { solution }}{\text { given } \mathrm{g} \text { solute }}\nonumber
\end{equation}
How many grams of clindamycin antibiotics are in a 45 mL capsule of the 1.0% (m/v) clindamycin?
Solution
Given: % m/v concentration: 1.08 % m/v clindamycin \(=\frac{1.0 \mathrm{~g} \text { clindamycin }}{100 \mathrm{~mL} \text { solution }}\), and volume of solution = 45 mL, Desired: ? g clindamycin?
Calculations: \begin{equation}
45 \text { mL solution } \times \frac{1.0 \mathrm{~g} \text { clindamycin }}{100 \mathrm{~mL} \text { solution }}=4.5 \mathrm{~g} \text { clindamycin. }\nonumber
\end{equation}
Parts per million (ppm) and parts per billion (ppb) concentration
Parts per million (ppm) is a number or ratio expressed as a fraction of a million (106).
For example, 2 ppm means \(\frac{2}{1,000,000}\) or 2:1,000,000, where 2 is the part, and 1,000,000 is the total. The concentration in ppm is calculated as a million times of part by total, i.e.: \begin{equation}
\text { Concentration in ppm }=\frac{p a r t}{\text { Total }} \times 10^{6}\nonumber
\end{equation}
Parts per billion (ppb) is a number or ratio expressed as a fraction of billion (109).
That is: \begin{equation}
\text { Concentration in ppb }=\frac{\text { part }}{\text { Total }} \times 10^{9}\nonumber
\end{equation}
Like percentage concentration, the ppm and ppb can be mass/mass (m/m), volume/volume (v/v) or mass/volume (m/v).
EPA’s action limit for copper is 1.3 mg/L in drinking water. What is this limit in ppm of copper m/v in the drinking water?
Solution
Given: 1.3 mg copper in 1L solution, Desired: ? ppm m/v of Copper in water
Formula: \(\text { Concentration in ppm }=\frac{\operatorname{solute}(\mathrm{~g})}{\operatorname{solution}(\mathrm{~mL})} \times 10^{6}\)
Calculations: First, convert the given units of mass and volume into the corresponding units that the formula takes, then plug the values in the formula and calculate.
\[\text { Solute }=1.3 \mathrm{~mg} \times \frac{1 \mathrm{~g}}{1000 \mathrm{~mg}}=0.0013 \mathrm{~g}\nonumber\]
\[\text { Solution }=1 \mathrm{~L} \times \frac{1000 \mathrm{~mL}}{1 \mathrm{~L}}=1000 \mathrm{~mL}\nonumber\]
\[\text { Concentration in ppm }=\frac{0.0013 \mathrm{~g}}{1000 \mathrm{~mL}} \times 10^{6}=1.3 \mathrm{~ppm} \text { copper } v / \mathrm{m}\nonumber\]
EPA’s action limit for lead is 0.015 mg/L in drinking water. What is this limit in ppb of lead m/v in the drinking water?
Solution
Given: 0.015 mg in 1L solution, Desired: ? ppb m/v of Lead in water
Formula: \(\text { Concentration in ppb }=\frac{\operatorname{solute}(g)}{\operatorname{solution}(\mathrm{mL})} \times 10^{9}\)
Calculations: First, convert the given units of mass and volume into the corresponding units that the formula takes, then plug the values in the formula and calculate.
\[\text { Solute }=0.015 \mathrm{~mg} \times \frac{1 \mathrm{~g}}{1000 \mathrm{~mg}}=0.000015 \mathrm{~g}\nonumber\]
\[\text { Solution }=1 \mathrm{~L} \times \frac{1000 \mathrm{~mL}}{1 \mathrm{~L}}=1000 \mathrm{~mL}\nonumber\]
\[\text { Concentration in ppb }=\frac{0.000015 \mathrm{~g}}{1000 \mathrm{~mL}} \times 10^{9}=15 \mathrm{~ppb} \text { lead } \mathrm{v} / \mathrm{m}\nonumber\]
Molarity
Molarity (M) expresses the moles of solute in a liter of solution.
The most common solution concentration unit used in chemistry is molarity (M):
\[\operatorname{Molarity}~({M})=\frac{n~(\text {moles of solute })}{V ~\text { (Litters of solution })}\nonumber\]
What is the molarity (M) of a solution prepared by dissolving 50.0 g NaOH in enough water to make 250 mL solution?
Solution
Given: Solute = 50.0 g NaOH, Volume of solution = 250 mL, Desired: ? M NaOH solution?
Formula: \(\text { Molarity }(M)=\frac{n~(\text {moles of solute })}{V~(\text {Litters of solution })}\)
Calculations: First, convert the given units of mass and volume into the corresponding units that the formula takes, then plug the values in the formula and calculate.
\[\text { Solute }=50.0 \mathrm{~g} \mathrm{~NaOH} \times \frac{1 \mathrm{~mol} \mathrm{~NaOH}}{40.00 \mathrm{~g} \mathrm{~NaOH}}=1.25 \mathrm{~mol} \mathrm{~NaOH}\nonumber\]
\[\text { Solution }=250 \mathrm{~mL} \times \frac{1 \mathrm{~L}}{1000 \mathrm{~mL}}=0.250 \mathrm{~L}\nonumber\]
\[\text { Molarity }(M)=\frac{1.25 \mathrm{~mol} \mathrm{~NaOH}}{0.250 \mathrm{~L} \mathrm{~solution}}=5.00 \mathrm{~M} \mathrm{~NaOH}\nonumber\]
The two conversion factor from molarity are the following:
\[\frac{n \text { (moles of solute) }}{V \text { (Litters of solution) }} \quad\text { and }\quad \frac{V \text { (Litters of solution) }}{n \text { (moles of solute })}\nonumber\]
How many litters of 0.211 M HCl solution are needed to provide 0.400 mol of HCl?
Solution
Given: amount of solute = 0.400 mol HCl, Concentration of solute = 0.211M = \(\frac{0.211 \text { mol HCl}}{1 L \text { solution }}\)
Calculations: \[\text { Liters of solution needed }=0.400 \mathrm{~mol} \mathrm{~HCl} \times \frac{1 \mathrm{~L} \text { soluton }}{0.211 \mathrm{~mol} \mathrm{~HCl}}=1.90 \mathrm{~L} \text { solution }\nonumber\]
A 2.50 L of 1.12 M NaOH solution contains how many moles of NaOH?
Solution
Given: Volume of solution = 2.50 L solution, concentration of solution = \(1.12 \mathrm{~M} \mathrm{~NaOH}=\frac{1.12 \mathrm{~mol} \mathrm{~NaOH}}{1 \mathrm{~L} \text { solution }}\), Desired: ? moles of NaOH?
Calculations: \[\text { Moles of } \mathrm{~NaOH} \text { in the solution }=2.50 \text { L solution } \times \frac{1.12 \mathrm{~mol} \mathrm{~NaOH}}{1 \mathrm{~L} \text { solution }}=2.80 \mathrm{~mol} \mathrm{} \mathrm{~NaOH}\nonumber\]
Dilution of solutions
Dilution of a solution is the addition of a solvent to decrease the solute concentration of the solute in the solution.
The product of concentration (C) and volume (V) is the amount of solute, i.e.,
\begin{equation}
\text { Amount of solute }=C \text { in } \frac{\text { amount of solute }}{\text { volume of solution }} \times V \text { in volume of solution. }\nonumber
\end{equation}
The amount of solute does not change by adding solvent. Therefore, the product of concentration and volume, i.e., CV, which is the amount of solute, is a constant, i.e.,
\begin{equation}
C_{1} V_{1}=C_{2} V_{2}=\text { amount of solute }\nonumber
\end{equation}
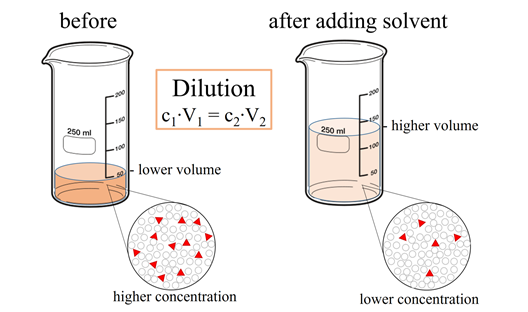
Fig. 5.4.3 shows that if the initial concentration is C1, the initial volume is V1, and after dilution, the final concentration is C2, the final volume is V2, then C1V1 = C2V2 = amount of solute that is constant. If three of the four variables in this equation are known, the missing one can be calculated, as explained in the following example.
Keep in mind that the concentrations and volumes should be in the same units on both sides of the equation: C1V1 = C2V2. If they are not in the same units, convert them to the same units before plunging them in the formula.
how much volume of 11.3 M HCl is needed to prepare 250 mL of 2.00 M HCl?
Solution
Given: C1 = 11.3 M HCl, C2 = 2.00 M HCl, V2 = 250 mL solution, Desired V1 = ?
Formula: C1V1 = C2V2, rearrange it to isolate the desired parameter: \(V_{1}=\frac{C_{2} V_{2}}{C_{1}}\)
Calculations: \[V_{1}=\frac{C_{2} V_{2}}{C_{1}}=\frac{2.00 \mathrm{~M~HCl} \times 250 \mathrm{~mL} \text { solution }}{11.3 \mathrm{~M} \mathrm{~HCl}}=44.2 \mathrm{~mL} \text { solution }\nonumber\]
what is the molarity of the NaOH solution prepared by diluting 100 mL of 0.521 M NaOH solution to 500 mL?
Solution
Given C1 = 0.521 M NaOH, V1 = 100 mL solution, V2 = 500 mL solution, Desired: Concentration of the final solution C2 = ? M NaOH
Formula: C1V1 = C2V2, rearrange to isolate the desired parameter: \(C_{2}=\frac{C_{1} V_{1}}{V_{2}}\)
Calculations: \[C_{2}=\frac{C_{1} V_{1}}{V_{2}} = \frac{0.521 \mathrm{~M~NaOH} \times 100 \mathrm{~mL} \mathrm{~NaOH}}{500 \mathrm{~mL} \mathrm{~NaOH}}=0.104 \mathrm{~M~NaOH}\nonumber\]
Dopamine is administered intravenously to a patient to increase blood pressure. How many milliliters (mL) of a 4.0% (m/v) dopamine solution is needed to prepare 250 mL of a 0.030% m/v) solution?
Solution
Given: C1 = 4.0% (m/v), C2 = 0.030% (m/v), V2 = 250 mL solution, Desired V1= ?
Formula: C1V1 = C2V2, rearrange to isolate the desired parameter: \(V_{1}=\frac{C_{2} V_{2}}{C_{1}}\)
Calculations: \[V_{1}=\frac{C_{2} V_{2}}{C_{1}}=\frac{0.030 \% \times 250 \mathrm{~mL} \text { solution }}{4.0 \%}=5.0 \mathrm{~mL} \text { of solution }\nonumber\]
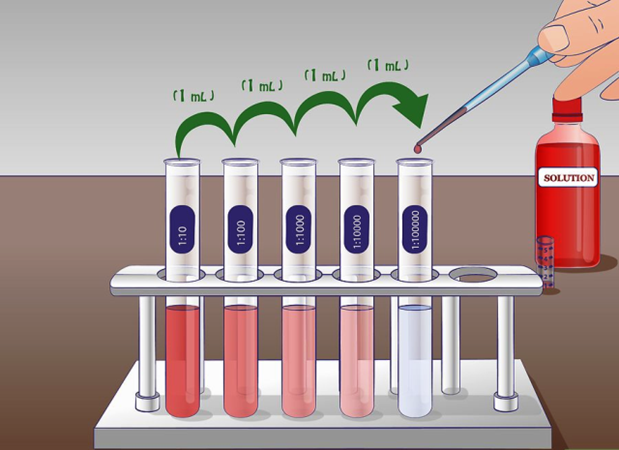
Logarithmic dilution
Take a unit volume of a given solution and add enough solvent to increase the volume of the solution 10 times for a logarithmic diluiton.
A logarithmic dilution is ten times dilution, i.e., proven by the following formula: \[C_{2}=\frac{C_{1} V_{1}}{V_{2}}=\frac{1 \mathrm{~mL} \times C_{1}}{10 \mathrm{~mL}}=0.1 \times C_{1}\nonumber\]
Repeating the above step with the diluted solution results in 10x10 = 100-time dilution, and repeating third-time results in 10x10x10 = 1000-time dilution. This dilution of 10 times in each step is called logarithmic dilution. Fig. 5.4.4 shows that five steps of logarithmic dilution on a 10% initial solution results in a concentration of 10 ppm in the final solution.