2.5: Electrons in atoms
- Page ID
- 372867
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The electronic structure of an atom determines the properties of the element. Knowledge of electromagnetic radiation is described first as it plays an essential role in understanding the electronic structure of atoms.
Electromagnetic radiations
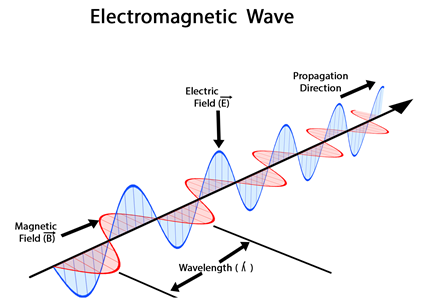
Electromagnetic radiations are waves that are oscillating electric and magnetic fields. The wave propagates in one direction, e.g., along the x-axis. The electric field oscillates perpendicular to it, e.g., along the y-axis. The magnetic field oscillates perpendicular to both, e.g., along the z-axis, as illustrated in Fig. 2.5.1. The distance between two consecutive maxima or between any two consecutive same phase points along the wave is called wavelength (\(\lambda\), pronounced ‘lambda’). The number of waves that pass a reference point in one second is called frequency (\(\nu\), pronounced ‘nu’). The speed of electromagnetic radiation is called the speed of light (c). The speed of light is the product wavelength and frequency, i.e.,
\[c = \lambda\nu\nonumber\]
The speed of light (c) is 3.00 x 108 m/s in a vacuum. The energy (E) of electromagnetic radiation is directly proportional to frequency, i.e.,
\[E = h\nu\nonumber\]
, where h is a constant, called plank’s constant. Replacing \(\nu\) with \(\frac{c}{\lambda}\) shows that the energy is inversely proportional to the wavelength.
\[E = \frac{h c}{\lambda}\nonumber\]
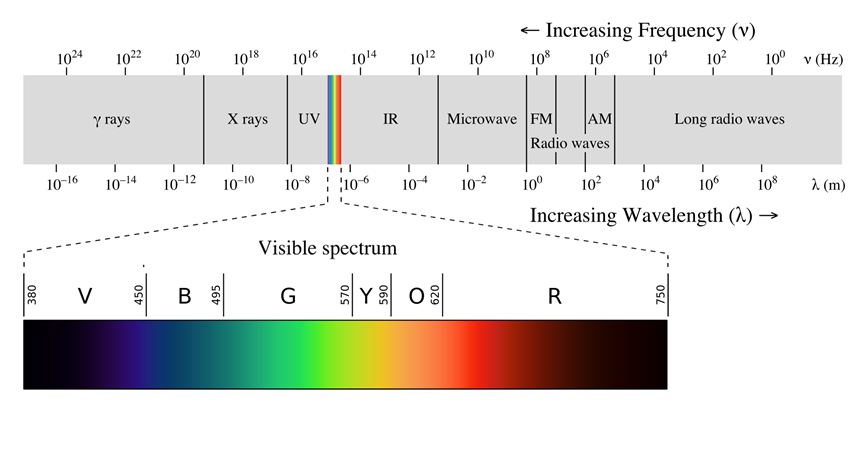
Fig. 2.5.2 illustrates the range of electromagnetic radiations that differ from each other concerning wavelength, frequency, or energy. The separation of the radiations based on their wavelength gives a spectrum. Visible light is a small portion of the spectrum of electromagnetic radiations, as illustrated in Fig. 2.5.2.
Continuous and line spectrum
The spectrum of sunlight contains radiations of all wavelengths within the visible range. The spectrum that contains all wavelengths in the range is called a continuous spectrum. Spectrum from sodium lamps or emissions from other atoms contains only some discrete wavelengths. The spectrum that contains discrete wavelengths is called the line spectrum. For example, as illustrated in Fig. 2.5.3, the emission from the hydrogen atom is a line spectrum.


Energy levels of electrons in an atom
The question is why the atomic emission has a discrete wavelength or discrete energy. The answer to this question came from the discoveries that concluded that the electron in an atom does not have continuous energy values; they have discrete energy values called shells and subshells.
The shell
Quantum numbers determine the allowed energy values of an electron in an atom.
- Principal quantum number (n) can have any integer value starting from 1, i.e., 1, 2, 3, 4, and so on.
- The smaller the n, the lower is the energy state, and the closer the electron is to the nucleus, the more tightly held the electron is by the nucleus.
- The value of n defines the shell, i.e., 1st shell has n = 1, 2nd shell has n = 2, 3rd shell has n =3, and so on.
Bohr introduced this concept of quantization of electronic energy levels. Fig. 2.5.4 illustrates Bohr’s atomic model.
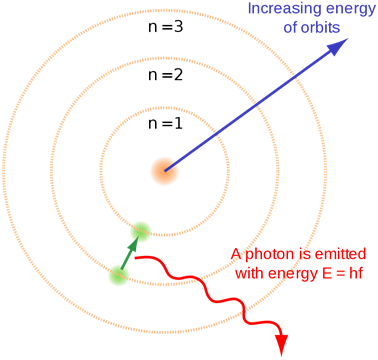
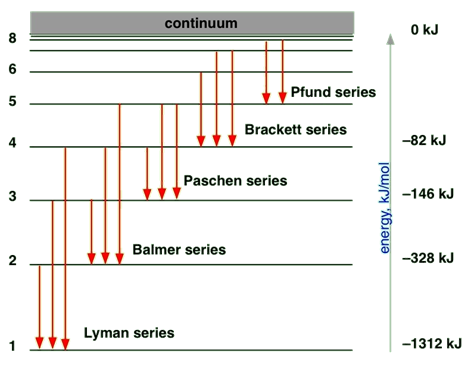
- When an electron jumps from a lower shell to a higher shell, it absorbs electromagnetic radiation of energy equal to the energy gap between the initial and the final shell.
- When an electron jumps from a higher shell to a lower shell, it emits radiation equal to the energy gap between the initial and the final shell.
Fig. 2.5.3 illustrates the emission of radiation from atoms –it is a line spectrum because only discrete energy levels, called shells, are allowed to electrons in an atom.
The subshell
A second quantum number, called Azimuthal quantum number (l) defines subshells within a shell.
The subshells are usually designated as s, p, d, f, ...
Each shell has subshells equal to the shell number. For example, 1st shall have only one subshell, i.e., s. It is designated 1s, where the number is the principal quantum number, and the letter is the subshell. The 2nd shell has two subshells 2s and 2p; the 3rd shell has three subshells 3s, 3p, and 3d; and the 4th shell has four subshells 4s, 4p, 4d, and 4f.
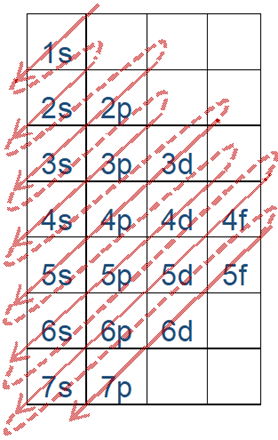
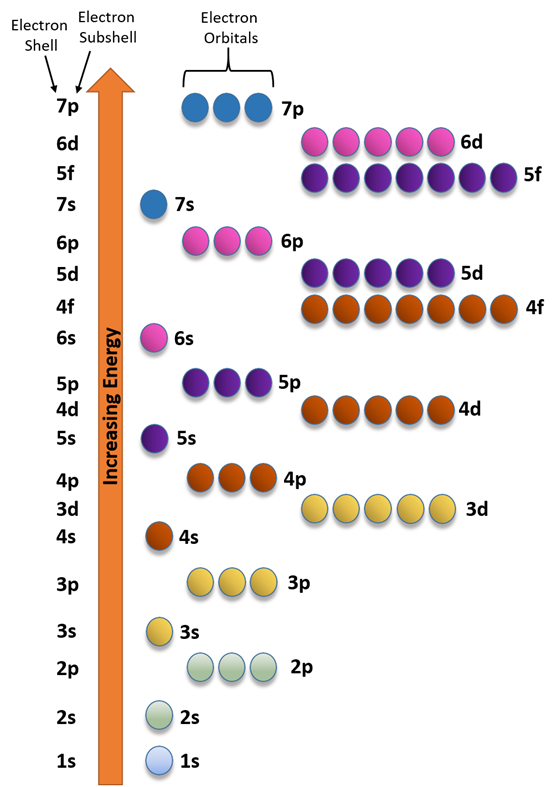
The energy order of subshells is 1s<2s<2p<3s<3p<4s and so on.
Fig. 2.5.6 helps in remembering the order. This figure is drawn by placing the orbitals in columns and shell numbers in rows in increasing order of n from top to bottom, starting from 1s orbitals in the first column and first row, p orbitals in the second, d in the third, and f in the fourth. The filling of electrons follows arrows going from corner to corner, starting from the top left corner of the topmost cell, as described in more detail in a later section.
The orbital is the region in space around the nucleus of an atom where electrons are most likely found.
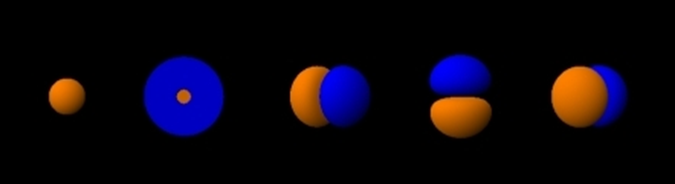
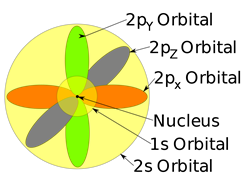
Each subshell has a certain number of orbitals in them. The orbitals have a specific shape and orientation. The s subshell has only one orbital spherically symmetrical, like a ball with a nucleus at the center. The 1s orbital is smaller than 2s, and 2s is smaller than 3s, but they all have a spherically symmetrical shape, as illustrated in Fig. 2.5.5. The p subshell has three orbitals. Each p orbital is a dumbbell shape with two lobes, i.e., px oriented along the x-axis, py along the y-axis, and pz along the z-axis, as illustrated in Fig. 2.5.5.
A set of orbitals having the same energy is called degenerate orbitals.
All the three p orbitals in the same shell have the same energy, i,e, 2px, 2py, and 2pz is a set of degenerate orbitals. The d subshell has five degenerate orbitals, and f subshell has seven degenerate orbitals, as shown in Fig. 2.5.7. Their shapes and orientations are more complex and not shown here.
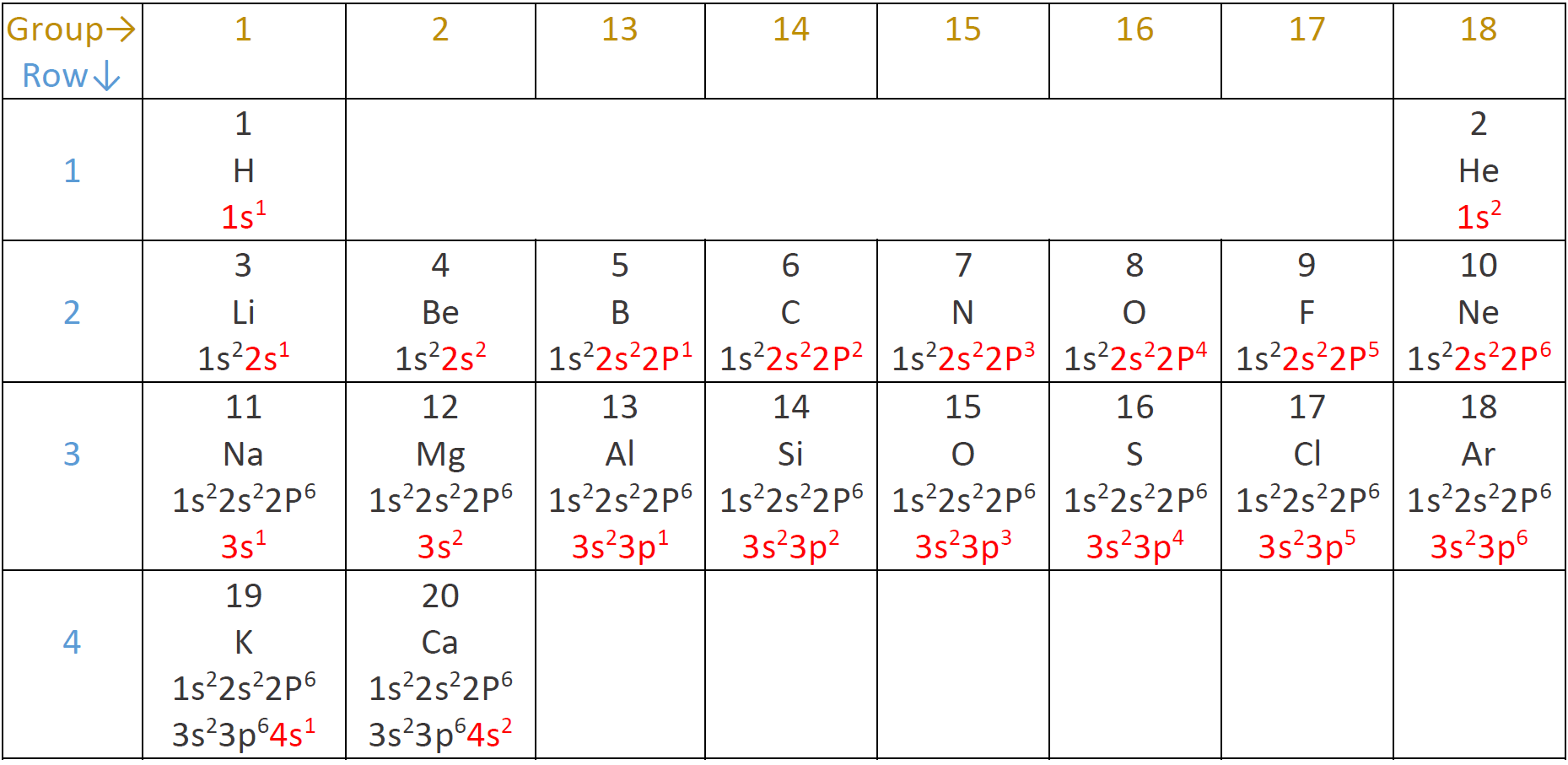
The electron configuration of atoms
The electron configuration is the distribution of electrons in the atom's orbitals.
- Each orbital can take a maximum of two electrons.
- The orbitals are filled in the order of increasing energy: the lowest energy orbital is filled with electrons before the next higher energy orbital starts filling up.
The energy order of orbitals is 1s<2s<2p<3s<3p<4s and so on, as shown in Fig. 2.5.6 and Fig. 2.5.7.
The electron configuration of 1st-row elements
The hydrogen atom has one electron, and it occupies 1s orbital, i.e., 1s1 represents the electron configuration of the hydrogen atom. The superscript in 1s1 shows the number of electrons in the sub-shell. A helium atom has two electrons, and both occupy 1s orbital, giving helium the electron configuration of 1s2. Hydrogen and helium are in the 1st-row of the periodic table and have the 1st-shell containing electrons.
The electron configuration of 2nd-row elements
The 2nd row starts from lithium with 3 electrons: the first two occupy 1s, and the third occupy 2s giving lithium the electron configuration 1s2 2s1. The next element is beryllium with 4 electrons with the configuration 1s2 2s2. The next element is a boron with 5 electrons and the electron configuration 1s2 2s2 2p1. Carbon has 6 electrons with the electron configuration 1s2 2s2 2p2. Remember that the s subshell has one orbital and can take a maximum of two electrons, but the p subshell has three orbitals and can take a maximum of six electrons, i.e., two per orbital. Nitrogen, oxygen, fluorine and neon have 7, 8, 9, and 10 electrons and have the electron configuration 1s22s22p3, 1s22s22p4, 1s22s22p5, and 1s22s22p6, respectively. Atomic number 3 to 10, i.e., lithium to neon, completes the 2nd-row.
The outermost shell is called the valence shell, and electrons in the valence shell are called valence electrons. 1st shell is the valence shell for 1st-row elements hydrogen and helium. 2nd-row elements have an inner shell with configuration 1s2, and a valence shell containing 2s and 2p being filled. The inner shell is also called the core-shell, and the electrons in the core-shell are called the core electrons.
The electron configuration of 3rd-row and 4th-row elements
The 3rd-row starts with sodium atomic number 11 and ends with argon atomic number 18. The electron configuration of the 3rd-row elements repeats the pattern of the 2nd-row, i.e., a set of full core-shells 1s22s22p6, and a valence shell having 3s or 3p being filled. The first two elements of the 4th-row are potassium and calcium with atomic numbers 19 and 20 having configurations 1s22s22p63s23p64s1 and 1s22s22p63s23p64s2, respectively. The electron configuration of elements with atomic numbers beyond 20 is more complicated, involving d and f subshell, and they are out of the scope of this book. Fig. 2.5.8 shows the electron configuration of the first twenty elements described above.