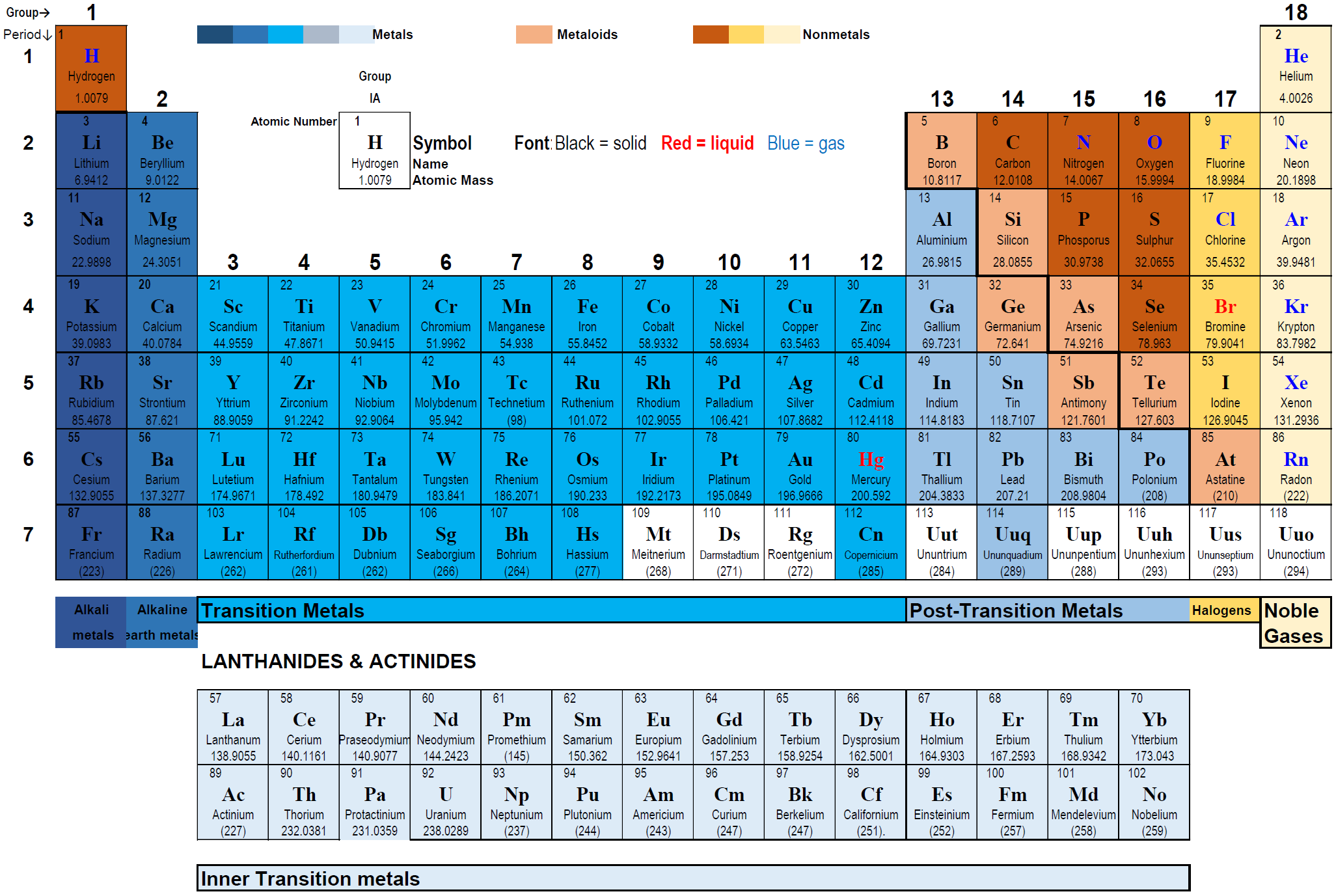
2.4: The periodic table
- Page ID
- 372866
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Early developments
The discoveries of elements happened over a long time. As the list of known elements grew, scientists tried to arrange them based on their properties. Mendeleev arranged elements in a table based on atomic masses. It resulted in elements having similar properties placed next to each other in most cases. There were gaps intentionally left in the table for the elements that were predicted based on the knowledge from the periodic table but not yet discovered.
Few exceptions were there observed where the properties of the elements did not agree with the group in which they were placed based on their atomic masses. Mosely developed a method to measure atomic numbers based on X-ray spectroscopy. The arrangement of the elements based on the atomic number instead of atomic masses removed the discrepancies in Mendeleev’s periodic table.
The modern periodic table of elements
- The modern periodic table of elements arranges the elements according to the increasing order of the atomic number starting from atomic number 1 for H and ending with atomic number 118 for Og, as shown in Fig. 2.4.1.
- The elements are arranged in horizontal rows called periods and vertical columns called groups.
Periods
The periodic table has seven horizontal rows called periods. The periods are numbered: 1 at the top to 7 at the bottom.
- The 1st period has only two elements: hydrogen in group 1 and helium in group 18, with a gap from group 2 to group 17.
- The 2nd and the 3rd periods have eight elements each, filling groups 1 and 2 followed by groups 13 to 18, leaving a gap from group 3 to group 12.
- The periods 4th and 5th periods have eighteen elements that are filled successively from group 1 to group 18.
- The periods 6th and 7th have 32 elements, each: the first two in groups 1 and 2, the next fourteen elements in separate rows below the table. These two rows of 14 elements each are called Actinides and Lanthanoids, respectively. Then the next sixteen elements fill the groups 3 to 18.
Groups
The periodic table has 18 vertical columns called groups or families. The groups are numbed starting from 1 on the leftmost and going through to 18 at the rightmost.
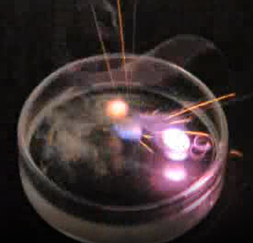
The 1st group is called alkali metals. The alkali metals include lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr), shown in Fig. 2.4.2. The alkali metals are the most reactive among the metals in the periodic table. They react vigorously with water, as shown in Fig. 2.4.3.





Figure \(\PageIndex{2}\): Alkali metals, from left to right: lithium, sodium, potassium, rubidium, and cesium. Source: Tomihahndorf at German Wikipedia, Dnn87 Contact email: Dnn87@yahoo.dk, and http://images-of-elements.com/potassium.php.
Hydrogen is in group 1 but is not included in alkaline earth metals. Hydrogen is a nonmetal and has properties quite different from alkali metals or any other group of elements.
The 2nd group is called alkaline earth metals. It includes beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). They are reactive metals but less reactive than alkali metals. Alkaline earth metals impart characteristic color to a flame. Salts of alkali metals are used in firework formulation to give distinctive colors to the firework, as shown in Fig. 2.4.4.

Groups 3 to 12 are called transition metals. They include precious metals like gold, silver, platinum, and construction metals like iron. Some make catalysts and are found in enzymes and other bio-molecules, like hemoglobin and chlorophyll.
Group 13 to group 16 does not have a unique name. They comprise nonmetals at the top and metals at the bottom of each group called post-transition metals. Important nonmetals include carbon, nitrogen, oxygen, phosphorous, and sulfur.
Group 17 elements are called halogens. The halogens include fluorine (F), chlorine (Cl), bromine, iodine (I), and astatine (At). The halogens are highly reactive nonmetals. Chlorine is gas, bromine is liquid, and iodine is solid at room temperature, as shown in Fig. 2.4.5.

Group 18 is called noble gases. They include helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). They are the least reactive of all the elements. Noble gases are used to create an inert atmosphere for chemical reactions. Noble gases are also used in the lighting system because of their chemically inert nature , as illustrated in Fig. 2.4.6.

Metals, metalloids, and nonmetals
Metals
The elements towards the right-bottom corner of the periodic table are metals except for hydrogen, which is a nonmetal. Metals have common characteristics, including:
- they are shiny,
- solid at room temperature (except mercury which is liquid),
- malleable (can be hammered into sheets) and ductile (can be drawn into wires),
- good conductors of heat and electricity, and
- tend to lose electrons and form ionic compounds when they react with nonmetals.
The metals of group 1 are called alkali metals; group 2 are called alkaline earth metals, group 3 to group 13 are called transition metals, the two-row below transition metals in the periodic table are called lanthanoids, and actinides or inner transition metals, and metals in the group 13 to group 16 are called post-transition metals. Elements other than transition or inner-transition metals, i.e., the elements of groups 1 and 2 and groups 13 to 18, are collectively called main group elements or representative elements.
Metalloids
The dividing line between metals and nonmetals is a staircase line starting from \(\ce{_5B}\) and ending at \(\ce{_85At}\). The elements on the staircase line are metalloids except for aluminum and polonium, which are considered metals. Metalloids have properties in-between metals and nonmetals; e.g., they have moderate heat and electrical conductivity.
Nonmetals
Elements towards the top-right corner of the periodic table and hydrogen are called nonmetals.
- The nonmetals usually have properties opposite to metals, e.g., they are not typically shiny, brittle if solid, and poor conductors of heat and electricity.
- Nonmetals tend to make ionic compounds by accepting electrons from metals and making molecular compounds by reacting with each other.
Two groups in nonmetals also have unique names, i.e., group 17 is called halogens, and group 18 is called Noble gases.
Generally, alkali metals are the most reactive, followed by alkaline earth metals, and halogens are the most reactive nonmetals. Noble gases are the least reactive nonmetals, also called inert gases.
Fig. 2.4.7 shows examples of a metal, a metalloid, and a nonmetal.



Elements essential for life
The elements that are the main constituents of humans and other living organisms are oxygen, carbon, hydrogen, nitrogen, and sulfur. Phosphorous is present in bone, teeth, and DNA. Calcium and magnesium are the main constituents of bones and teeth and perform some other body functions. Sodium and potassium cations are the main electrolytes in body fluids, and chloride anion balances the charge. Iron is present in hemoglobin that carries oxygen to the cells. These elements are essential to life, and they are macronutrients. Besides these, several other elements are needed in a small amount. They are essential for life and called micronutrients. Fig. 2.4.8 shows the macronutrients in pink and micronutrients in blue color in a periodic table.

Physical and chemical states of elements
Elements in gases state at ambient conditions
The term molecule is generally used for an electrically neutral group of two or more atoms held together by chemical bonds. In the kinetic molecular theory of gases, a molecule is the smallest particle of an element or compound with a stable and independent existence. Atoms of noble gases exist as independent species in the gas phase at room temperature, i.e., as monoatomic molecules like He, Ne, Ar, Kr, Xe, and Rn. Other elements that are gases at room temperature or diatomic molecules, i.e., H2, N2, O2, F2, and Cl2, are called dihydrogen, dinitrogen, dioxygen, and dichlorine, respectively. Note that usually prefix di is not used for the name of the element, i.e., these are usually called hydrogen, nitrogen, oxygen, fluorine, and chlorine, respectively. Monoatomic species of these elements, i.e., H, N, O, F, and Cl, exist, but they are very reactive species called free radicals, and they do not survive for a long time.
Elements in the liquid state at ambient conditions
Two elements exist as liquids at room temperature, i.e., mercury (Hg), a metal, and bromine (Br2), a no metal that exists as diatomic molecules. Four elements, francium, cesium, gallium, and rubidium, are solid metals at 25 oC, but become liquid when the temperature is slightly warmer.
Elements in the solid-state at ambient conditions
All elements not mentioned in the previous two sections are solid at room temperature. They may be diatomic or polyatomic molecules, e.g., I2, O3, P4, S8, diiodine, ozone, tetraphosphorous, and octasulfur, with two or three, four, and eight atoms, respectively, in a molecule.
Allotropes
Different forms of the same element in the same physical state are called allotropes.
The allotropes are different structural modifications of the element. For example, carbon exists in several allotropic forms; two of them are shown in Fig. 2.4.9. Another example is O2, and O3 are gaseous forms of oxygen.

Elements that exist as giant molecules
Some elements exist as giant molecules, i.e., a collection of many atoms bonded with each other through a 3D network of bonds. For example, carbon is a giant molecule in several allotropic forms, including diamond, graphite, carbon nanotubes, and fullerenes. Fig. 2.4.9 shows the bonding in diamond and graphite allotropes. The whole piece of diamond is one molecule with carbon atoms interconnected in a 3D network of bonds.
Metals also exist as a collection of many atoms bonded together by metallic bonds. The metal atoms exist as +ions arranged in a well-defined 3D arrangement called crystal lattice with some of the outermost electrons roaming around in the whole piece of the metal, forming a sea of electrons around the metal atoms, as illustrated in Fig. 2.4.10.
The elements in giant molecules and metals are represented by element symbols without any subscript, e.g., C is carbon, Fe is iron, Au is gold, etc.



