2.3: Atoms of elements
- Page ID
- 372865
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Symbol of an element
Elements are represented by symbols, the first alphabet of their English or non-English name, written in capital letters. For example, C for carbon, N for nitrogen, and I for iodine. Usually, another alphabet is also chosen from the element's name and written as a small letter, e.g., Al for aluminum, Zn for zinc, and Ca for calcium. Some element symbols are derived from non-English names, e.g., Na for sodium from the Latin name natium, Cu for copper from Latin cupurum, and Ag for silver from Latin argentum.
Atoms of the same element have the same number of protons, and atoms of different elements have different numbers of protons. In other words, the number of protons in an atom defines the element. There are 118 elements known at this time; the number of protons in atoms varies from 1 for hydrogen to 118 for Oganesson (Og).
The number of protons in an atom is called the atomic number.
The atomic number defines the element. A subscript to the left of the symbol of an element represents the atomic number. For example, \(\ce{ _1H}\) shows one proton in a hydrogen atom, and \(\ce{_6C}\) shows 6 protons in a carbon atom.
The number of protons plus the number of neutrons in an atom is the mass number.
A superscript to the left of the symbol of an element represents the mass number. For example, \(\ce{^1_1H}\) is a hydrogen atom with atomic number 1, mass number 1, and no neutrons, while \(\ce{^19_9F}\) is a fluorine atom with 9 protons and 10 neutrons.
The number of electrons in an atom equals the number of protons minus the charge on the atom.
The number of electrons is equal to the number of protons in the case of a neutral atom, as there is no charge on a neutral atom.
A neutral atom can lose some electrons and become a positively charged particle, called a cation.
The charge is represented as a superscript on the right side of the element symbol, e.g. \(\ce{^1_1H^+}\) is hydrogen without any electron, i.e., 1 proton, 0 neutrons, and 0 electrons. \(\ce{^20_40Ca^2+}\) is calcium with two fewer electrons than protons, i.e., 20 protons, 20 neutrons, and 18 electrons.
An atom can gain electrons and become a negatively charged particle, called an anion.
For example, \(\ce{^16_8O^2-}\) is oxygen with two more electrons than protons on it, i.e., 8 protons, 8 neutrons, and 10 electrons. A \(\ce{^19_9F^-}\) is fluorine with one more electron than protons on it, i.e., 9 protons, 10 neutrons, and 10 electrons. Fig. 2.3.1 illustrates the gain or loss of electrons from neutral atoms.
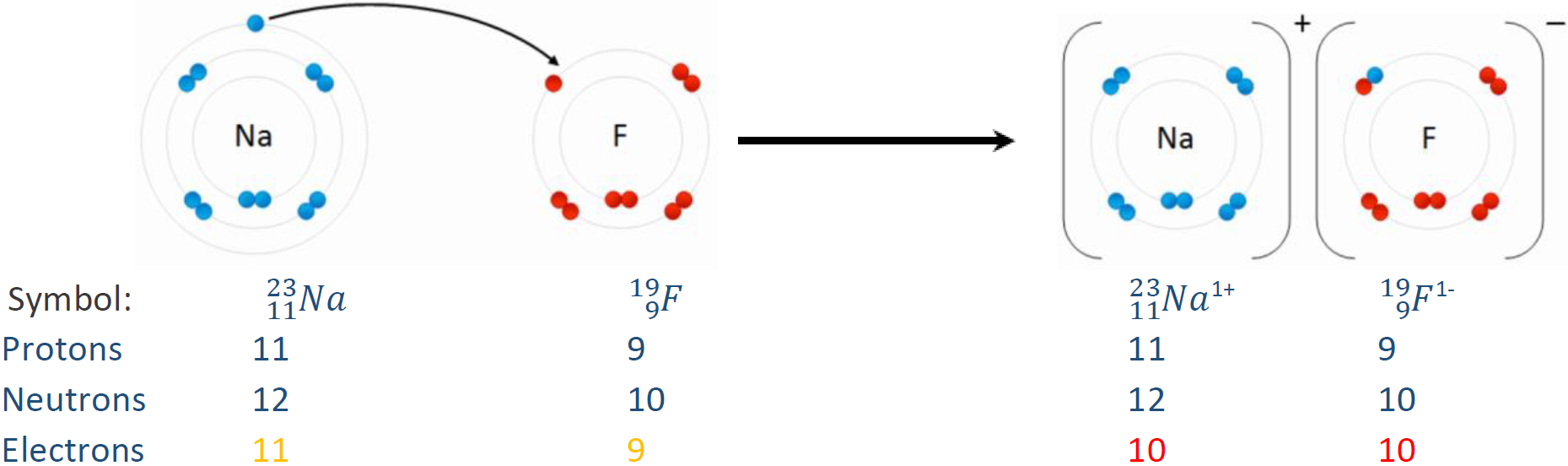
Calculating the number of protons, neutrons, and electrons
In general, an atom of a hypothetical element X is represented as \({ }_{Z}^{A} X^{I ~charge}\) where Z is the atomic number, A is the mass number, and I is an integer equal to charge number and charge the sign of the charge number: + or -.The number of protons, neutrons, and electrons is calculated by using the following formulas:
Number of protons = A,
Number of neutrons = A – Z, and
Number of electrons = Z – (charge I),
where a charge is a + or - sign of the charge number.
Calculate the number of protons, neutrons, and electrons in \(\ce{^16_8O}\), \(\ce{^16_8O^2-}\), and \(\ce{^16_8O^+}\) ?
Solution
\(\ce{^16_8O}\): number of protons = Z = 8, number of neutrons = A – Z = 16-8 = 8,
and number of electrons = Z – (charge I) = 8 – 0 = 8.
\(\ce{^16_8O^2-}\): number of protons = Z = 8, number of neutrons = A – Z = 16-8 = 8,
and number of electrons = Z – (charge I) = 8 – (-2) = 8 + 2 = 10.
\(\ce{^16_8O^1+}\): number of protons = Z = 8, number of neutrons = A – Z = 16-8 = 8,
and number of electrons = Z – (charge I) = 8 – (+1) = 8 – 1 = 9.
If charge number I is 1 in \({ }_{Z}^{A} X^{I ~charge}\), it is usually not written, but a number more than one is written. For example, \(\ce{^16_8O^-}\) has change = -1, \(\ce{^16_8O^2-}\) has charge charge -2, and \(\ce{^16_8O^+}\) has charge = +1.
Isotopes
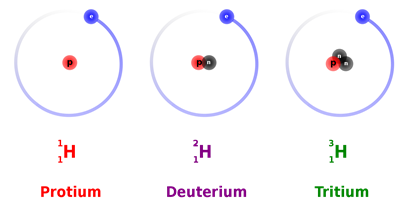
All atoms of the same element have the same number of protons but can have a different number of neutrons. For example, \(\ce{^1_1H}\), \(\ce{^2_1H}\), and \(\ce{^3_1H}\) have neutrons equal to 0, 1, and 2, respectively.
Atoms of the same element that have a different number of neutrons are called isotopes.
For examples, \(\ce{^1_1H}\), \(\ce{^2_1H}\), and \(\ce{^3_1H}\) are isotopes of hydrogen illustrated in Fig. 2.3.2. Another example is \(\ce{^6_3Li}\), and \(\ce{^7_3Li}\) are two isotopes of lithium. Natural samples of elements usually have almost constant ratios of isotopes. Table 1 lists some Isotopes of elements and their percent abundance in typical natural samples.
| Element | Isotopes | Abundance (%) |
|---|---|---|
| Hydrogen |
\(\ce{^1_1H}\) \(\ce{^2_1H}\) \(\ce{^3_1H}\) |
99.99 0.01 Negligible |
| Lithium |
\(\ce{^6_3Li}\) \(\ce{^7_3Li}\) |
7.6 92.4 |
| Carbon |
\(\ce{^12_6C}\) \(\ce{^13_6C}\) \(\ce{^14_6C}\) |
98.93 1.07 Negligible |
| Chlorine |
\(\ce{^35_17Cl}\) \(\ce{^37_17Cl}\) |
75.78 24.22 |
| Bromine |
\(\ce{^79_35Br}\) \(\ce{^81_35Br}\) |
50.69 49.31 |
| Uranium |
\(\ce{^235_92U}\) \(\ce{^238_92U}\) |
0.72 99.28 |
Atomic mass
The atomic mass listed in the periodic table is the weighted average of the masses of the isotopes present in a natural sample of the element.
The following formula calculates the atomic mass:
\[\text { Atomic mass }=\sum[(\text { mass of isotope }) \times(\text { fractional abuncance of the isotope })\nonumber\]
, where \(\sum\) means summation over all isotopes of the element, the fractional abundance of the isotope is the % abundance divided by 100. Fig. 2.3.3. illustrates how the atomic mass is listed in a periodic table.
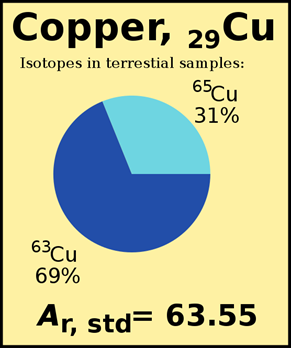
Calculate the atomic mass of chlorine with two isotopes in nature samples, i.e., \(\ce{^35_17Cl}\) of mass 34.969 amu and % abundance 75.78% and \(\ce{^37_17Cl}\) of mass 36.996 amu and % abundance 24.22%.
Solution
Formula: \(\text { Atomic mass }=\sum[(\text { mass of isotope }) \times(\text { fractional abuncance of the isotope })\)
Plug in the given values in the formula and calculate:
\[\left(34.969 \mathrm{~amu} \times \frac{75.78}{100}\right)+\left(36.996 \mathrm{~amu} \times \frac{24.22}{100}\right)=35.45 \mathrm{~amu}\nonumber\]
- Atomic masses of isotopes are close to but not the same as their mass numbers. For example, \(\ce{^35_17Cl}\) has mass number mass = 35, but the atomic mass of this isotope is 34.969 amu as shown in the above example.
- The weighted average atomic mass is usually closer to the mass number of the most abundant isotope, e.g., 35.45 amu in the above example is close to the mass number 35 of \(\ce{^35_17Cl}\) isotope, which is the most abundant isotope.
- The periodic table reports the atomic mass as calculated in the above example, i.e., the weighted average of the masses of the isotopes present in the natural sample of the element.


