2.2: Subatomic particles and a modern view of an atom
- Page ID
- 372864
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Atoms are composed of fundamental subatomic particles: electrons, protons, and neutrons.
Electron was the first subatomic particle discovered. The discovery of electrons is related to the study of cathode rays and the basic knowledge of charges, i.e., there are two types of charges +ve and –ve; like charges repel each other; opposite charges attract each other as illustrated in Fig. 2.2.1; electric and magnetic field deflects the moving charges.
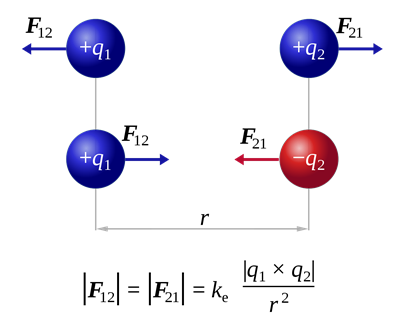
The discovery of the electron
Cathode rays
Cathode rays are a type of radiation emitted from a cathode (negatively charged electrode) when a high electric field is applied across a pair of electrodes under reduced pressure conditions, as illustrated in Fig. 2.2.2. The cathode rays travel in the inter-electrode space. If there is a hole in the anode, the cathode rays can pass through the hole and keep moving in a straight path. The cathode rays emit light when they strike a fluorescent screen.
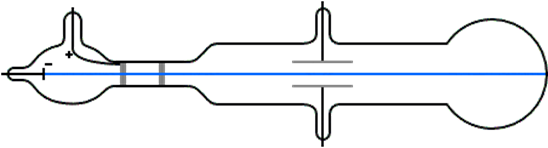
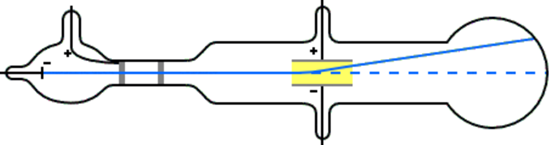
J.J Thomson's experiments -the discovery of electrons
J.J. Thomson studied the cathode rays and found that an electric field deflects the cathode rays towards the positive electrode. This observation indicates that the cathode rays were negative charges. Changing the cathode material did not change the properties of the cathode rays. J.J. Thomson concluded from these observations that the cathode rays were streams of particles, called electrons, that are present in the atoms of all elements.
Further discoveries revealed that the charge on the electrons is 1.602 x 10-19 C, and their mass is 9.10 x 10-28 g. The electrons are incredibly light, about two thousand times lighter than the lightest atom.
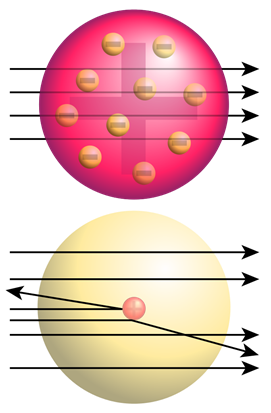
Plum Pudding Model of an atom
Based on the information from cathode ray experiments, J.J. Thomson concluded that there are –ve charge electrons and a +ve matter representing almost all the atom's mass. He proposed the plum-pudding model of atoms, i.e., the positive matter is like a diffused cloud or jelly that occupies the atom's space, and electrons are embedded in it like fruits in jelly in the case of the plum-pudding dessert dish as shown in Fig. 2.2.3.

The discovery of the nucleus of an atom
\({\alpha}\)-Rays
\({\alpha}\)-Rays, pronounced as alpha-rays, are high-energy radiations emitted from some radioactive sources. The \({\alpha}\)-rays are composed of \({\alpha}\)-particles that are helium atoms without any electrons.
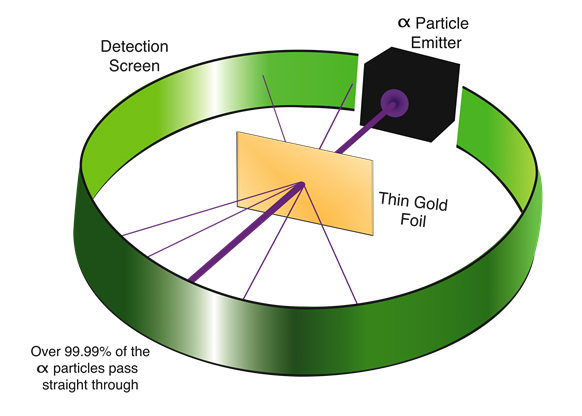
Rutherford's gold foil experiment -the discovery of the nucleus
Rutherford tested the plum pudding model of the atom by bombarding \({\alpha}\)-rays on a thin gold foil. He expected that \({\alpha}\)-particles to pass through the gold foil un-deflected like bullets fired through a Styrofoam sheet. He observed that although most of the \({\alpha}\)-particles passed through the gold-foil un-deflected, one in ~20,000 deflected at larger angles, as illustrated in Fig. 2.2.4.
Since the plum-pudding model of an atom could not explain the deflection of the \({\alpha}\)-particles, Rutherford concluded that there was a tiny but very dense region in the center of an atom, now called the nucleus, that deflected the \({\alpha}\)-particles. Rutherford’s gold foil experiment led to the discovery of the nucleus.
The discovery of proton
Rutherford predicted that a positively charged fundamental particle of atoms should reside in the nucleus. Later on, Rutherford observed that shining \({\alpha}\) -rays on nitrogen gas produced positively charged particles called protons. The protons are about 20,000 times heavier than electrons but carry a +ve charge equal in magnitude to the –ve charge on an electron.
The discovery of the neutron
The mass of protons and electrons did not account for an atom's overall mass, which led to a search for another subatomic particle. James Chadwick discovered that bombarding \({\alpha}\) -rays on a beryllium target produced a highly penetrating radiation consisting of a beam of neutral particles, now called neutrons. The presence of neutrons in the nucleus accounts for the missing mass of atoms.
Other sub-atomic particles have been discovered, e.g., quarks that constitute protons and neutrons, but their knowledge is not critical for understanding basic chemistry.
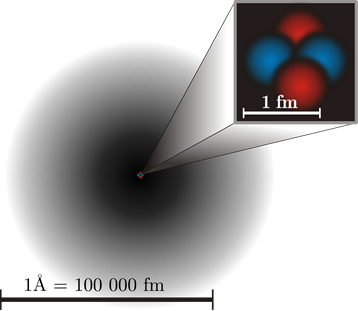
Modern view of an atom
Protons and neutrons reside in the nucleus with a diameter of 10-15 m. Electrons occupy the region outside the nucleus with a diameter of 10-10 m, as illustrated in Fig. 2.2.5. If the nucleus is about the size of a marble, the atom would be about the size of a soccer field.
Properties of the subatomic particles
The basic SI units of mass, electric charge, and distance are too big for atomic-scale measurements. New units are defined for this purpose, i.e.:
Atomic mass unit (amu) which is 1/12 of the mass of a single carbon atom that has 6 protons and 6 neutrons in it.
\[1 \mathrm{~amu}=1.660539606660(50) \times 10^{-27} \mathrm{~kg}\nonumber\]
Charge on one electrons is electron charge (e). \[1 ~e=1.602176634 \times 10^{-19} \mathrm{~C}\nonumber\]
1 Å = 10-10 m
These units are usually used for masses, charges, and diameters of atoms. Table 1 lists the basic properties of the subatomic particles.
| Particle | Charge (e) | Mass (amu) |
|---|---|---|
| Proton | +1 | 1.0073 |
| Neutron | 0 | 1.0078 |
| Electron | -1 | 5.486 x 10-4 |
How the subatomic particles are held in the atom
The gravitational force is negligible in the case of the behavior of subatomic particles in the atoms. Although electrons repel other electrons, they stay in a tiny space due to attraction towards the nucleus. Similarly, protons repel each other, but the electrical force is small compared to the strong nuclear force that holds protons and neutrons together in the nucleus.


