1.7: Density and specific gravity measurements
- Page ID
- 372554
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Density
Density is the mass-to-volume ratio of a substance.
Density is a physical characteristic of matter. Each substance has a characteristic density that can be used as one hint in identifying a substance.
Gases have very low density, usually expressed in g/L. For example, air density is around 1.224 g/L at sea level and 15 oC. The density of liquids and solids is usually expressed in g/mL. For example, the density of water at 4 oC is 1.00 g/mL.
Objects that are less dense than water float, and the denser objects than water sink in the water. For example, oil is less dense than water and floats on water. Metals are denser than water and sink in water. The density of some common substances is listed in Table 1
| Substance | Density (g/mL) |
|---|---|
| hydrogen | 0.000089 |
| carbon dioxide | 0.0019 |
| ethyl alcohol | 0.7893 |
| water | 1.00 |
| magnesium | 1.74 |
| table salt | 2.16 |
| aluminum | 2.70 |
| iron | 7.86 |
| copper | 8.92 |
| silver | 10.50 |
| lead | 11.34 |
| mercury | 13.59 |
| gold | 19.30 |
Density measurement
Density (d) is calculated from the mass (m) and volume (V) of a substance by the formula:
\[d = \frac{m}{V}\nonumber\]
Mass is usually measured using an analytical balance. The volume of liquids can be measured using a graduated cylinder, pipet, or density bottle. The volume of regular solids can be calculated from the geometric parameters. For example, the volume of a rectangle is equal to length x width x height. The volume of a cube is equal to the edge length cubed.
The volume of an irregular shaped sold is usually measured because the substances that are denser than water sink and displace an equal amount of water. Fig. 1.7.1 illustrates the density measurement of an irregular-shaped solid object that sinks in water, as explained in the following example.
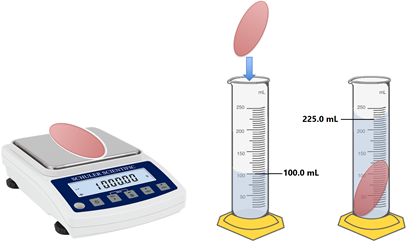
Wwhat the density of the object in Fig. 1.7.1?
Soution
the mass (m) of the object on balance is 1000.00 g. The volume of the object is equal to the volume of the water displaced by the object, which is 225.0 mL – 100.0 mL = 125.0 mL.
\begin{equation}
d=\frac{m}{V}=\frac{1000.00 \mathrm{~g}}{125.0 \mathrm{~mL}}=8.000 \frac{\mathrm{g}}{\mathrm{ml}}\nonumber
\end{equation}
Most of the time, Calculators give more significant numbers and sometimes less than needed; both need correction. In example 1.7.1, the calculator displays 8, i.e., one significant figure, but three zeros are added to make four significant figures.
Bone density and osteoporosis
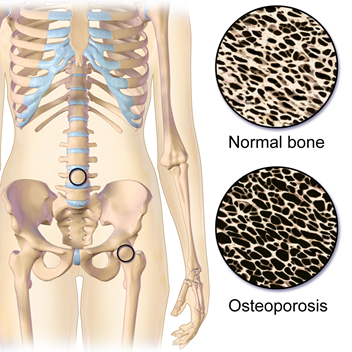
Osteoporosis is a bone disease associated with decreased bone density, particularly in older adults. Bones always lose and gain calcium, magnesium, and phosphate. In childhood, the bones build faster than decay, but in old age, the process reverses, and the bones start to thin, loos strength, and become more prone to fracture, as illustrated in Fig. 1.7.2. Hormonal changes, diseases, and some medications contribute to bone thinning. Severe loss of bone density is called osteoporosis.
Specific gravity
Specific gravity is the ratio of the object's density to the density of water, i.e.:
\begin{equation}
\text { Specific gravity }=\frac{\text { Density of an object }}{\text { Density of water }}\nonumber
\end{equation}
Specific gravity is the ratio of the object's density to the density of water, i.e.:
\begin{equation}
\text { Specific gravity }=\frac{\text { Density of an object }}{\text { Density of water }}\nonumber
\end{equation}
The units cancel out in the ratio. Therefore, the specific gravity is a unitless number. The density of water is 1.0 g/mL at room temperature, so the specific gravity is equal to the density of the object expressed without a unit.
When substances dissolve in water, the density of the solution is usually different from pure water. For example, the density of whole blood for humans is ~1.060 g/mL. The density of urine varies in the range of 1.0050 g/mL to 1.030 g/mL. Both the blood and urine have dissolved substances in water that increase the density from that of pure water. Both high and low density or specific gravity than the normal range of urine indicates medical problems. An increase in the specific gravity of urine indicates that it is due to an increase in the solutes caused by dehydration, diarrhea, or infection. Similarly, a decrease in solute concentration decreases the specific gravity of urine, which indicates medical problems like renal failure.
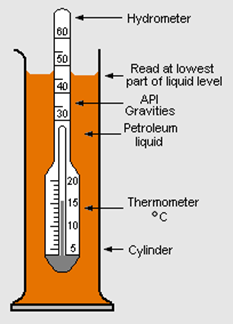
Specific gravity measurement
The specific gravity is usually measured using an instrument called a hydrometer. The hydrometer partially submerges in the liquid sample, and the reading on the scale at the air-water junction point is recorded, as illustrated in Fig. 1.7.3.