11.6: Penetrating Power of Radiation
- Page ID
- 152208
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Compare qualitatively the ionizing and penetration power of alpha particles \(\left( \alpha \right)\), beta particles \(\left( \beta \right)\), and gamma rays \(\left( \gamma \right)\).
With all the radiation from natural and man-made sources, we should quite reasonably be concerned about how all the radiation might affect our health. The damage to living systems is done by radioactive emissions when the particles or rays strike tissue, cells, or molecules and alter them. These interactions can alter molecular structure and function; cells no longer carry out their proper function and molecules, such as DNA, no longer carry the appropriate information. Large amounts of radiation are very dangerous, even deadly. In most cases, radiation will damage a single (or very small number) of cells by breaking the cell wall or otherwise preventing a cell from reproducing.
The ability of radiation to damage molecules is analyzed in terms of what is called ionizing power. When a radiation particle interacts with atoms, the interaction can cause the atom to lose electrons and thus become ionized. The greater the likelihood that damage will occur by an interaction is the ionizing power of the radiation.
Much of the threat from radiation is involved with the ease or difficulty of protecting oneself from the particles. How thick of wall do you need to hide behind to be safe? The ability of each type of radiation to pass through matter is expressed in terms of penetration power. The more material the radiation can pass through, the greater the penetration power and the more dangerous they are. In general, the greater mass present the greater the ionizing power and the lower the penetration power.
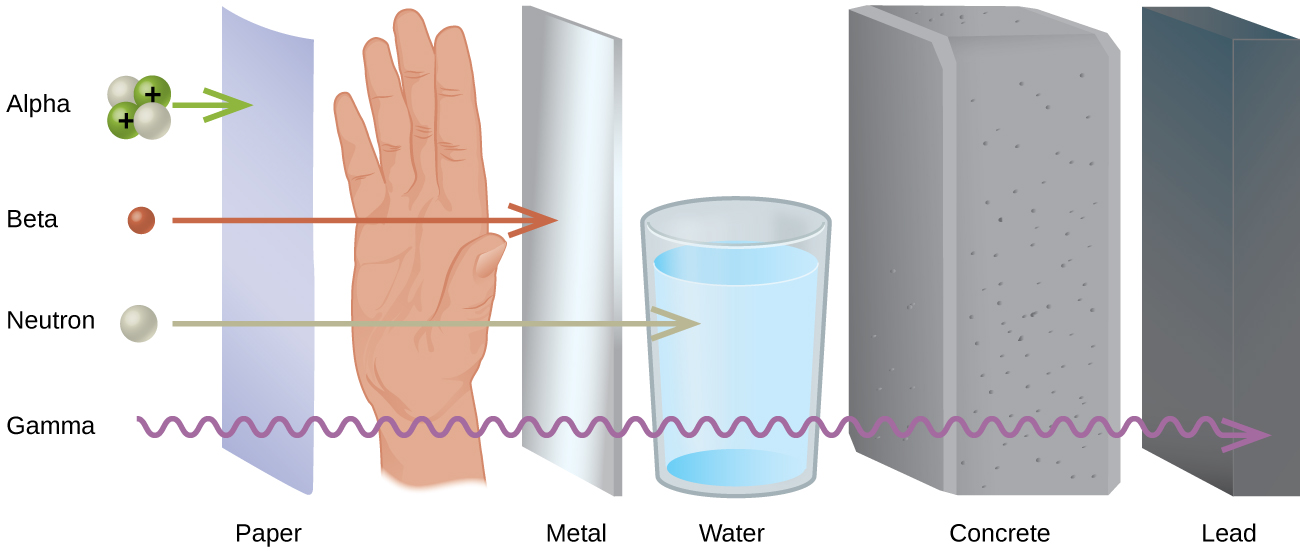
Comparing only the three common types of ionizing radiation, alpha particles have the greatest mass. Because of the large mass of the alpha particle, it has the highest ionizing power and the greatest ability to damage tissue. That same large size of alpha particles, however, makes them less able to penetrate matter. Alpha particles have the least penetration power and can be stopped by a thick sheet of paper or even a layer of clothes. They are also stopped by the outer layer of dead skin on people. This may seem to remove the threat from alpha particles but only from external sources. In a situation like a nuclear explosion or some sort of nuclear accident where radioactive emitters are spread around in the environment, the emitters can be inhaled or taken in with food or water and once the alpha emitter is inside you, you have no protection at all.
Beta particles are much smaller than alpha particles and therefore, have much less ionizing power (less ability to damage tissue), but their small size gives them much greater penetration power. Most resources say that beta particles can be stopped by a one-quarter inch thick sheet of aluminum. Once again, however, the greatest danger occurs when the beta emitting source gets inside of you.
Gamma rays are not particles but a high energy form of electromagnetic radiation (like x-rays except more powerful). Gamma rays are energy that has no mass or charge. Gamma rays have tremendous penetration power and require several inches of dense material (like lead) to shield them. Gamma rays may pass all the way through a human body without striking anything. They are considered to have the least ionizing power and the greatest penetration power.
A comparison of Alpha particles, beta particles and gamma rays is given in Table \(\PageIndex{1}\).
| Particle | Symbol | Mass | Penetrating Power | Ionizing Power | Shielding |
|---|---|---|---|---|---|
| Alpha | \(\alpha\) | \(4 \mathrm{amu}\) | Very Low | Very High | Paper Skin |
| Beta | \(\beta\) | \(1 / 2000 \mathrm{amu}\) | Intermediate | Intermediate | Aluminum |
| Gamma | \(\gamma\) | 0 (energy only) | Very High | Very Low | 2 inches lead |
The safest amount of radiation to the human body is zero. It isn't possible to be exposed to no ionizing radiation so the next best goal is to be exposed to as little as possible. The two best ways to minimize exposure is to limit time of exposure and to increase distance from the source.
Summary
- Types of radiation differ in their ability to penetrate material and damage tissue, with alpha particles the least penetrating but potentially most damaging and gamma rays the most penetrating.
- The two best ways to minimize exposure is to limit time of exposure and to increase distance from the source.

