3.6: Electron Arrangement- The Bohr Model (Orbits)
- Page ID
- 152152
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Know the properties of different types of electromagnetic radiation.
- Define an energy level in terms of the Bohr model.
- Discuss how the Bohr model can be used to explain atomic spectra.
- Describe the arrangement of electrons using the shell model.
Electromagnetic waves have an extremely wide range of wavelengths, frequencies, and energies. The highest energy form of electromagnetic waves are gamma (γ) rays and the lowest energy form are radio waves.
The figure below shows the electromagnetic spectrum, which is all forms of electromagnetic radiation. On the far left of Figure \(\PageIndex{1}\) are the highest energy electromagnetic waves. These are called gamma rays and can be quite dangerous, in large numbers, to living systems. The next lower energy form of electromagnetic waves are called x-rays. Most of you are familiar with the penetration abilities of these waves. They can also be dangerous to living systems. Humans are advised to limit as much as possible the number of medical x-rays they have per year. Next lower, in energy, are ultraviolet rays. These rays are part of sunlight and the upper end of the ultraviolet range can cause sunburn and perhaps skin cancer. The tiny section next in the spectrum is the visible range of light … this section has been greatly expanded in the bottom half of the figure so it can be discussed in more detail. The visible range of electromagnetic radiation are the frequencies to which the human eye responds. Lower in the spectrum are infrared rays and radio waves.
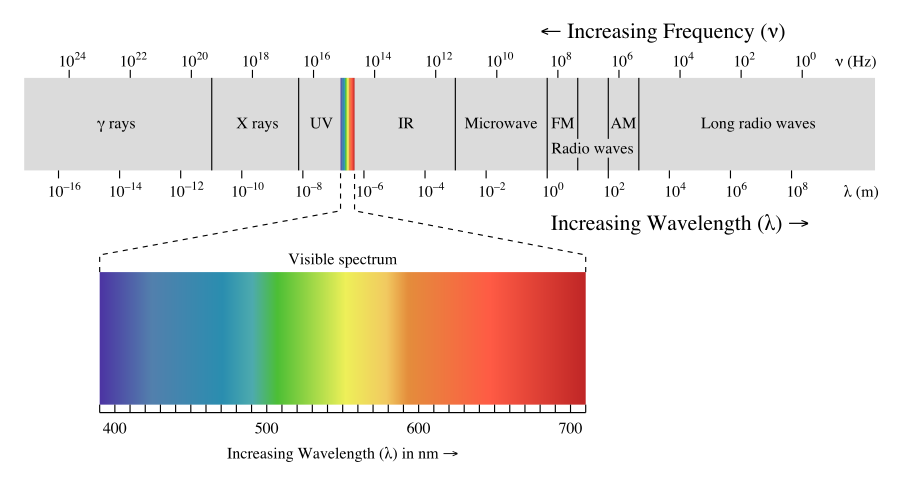
The light energies that are in the visible range are electromagnetic waves that cause the human eye to respond when those frequencies enter the eye. The eye sends a signal to the brain and the individual "sees" various colors. The highest energy waves in the visible region cause the brain to see violet and as the energy decreases, the colors change to blue, green, yellow, orange, and red. When the energy of the wave is above or below the visible range, the eye does not respond to them. When the eye receives several different frequencies at the same time, the colors are blended by the brain. If all frequencies of light strike the eye together, the brain sees white and if there are no visible frequencies striking the eye, the brain sees black. The objects that you see around you are light absorbers - that is, the chemicals on the surface of the object will absorb certain frequencies and not others. Your eyes detect the frequencies that strike your eye. Therefore, if your friend is wearing a red shirt, it means the dye in that shirt absorbs every frequency except red and the red frequencies are reflected. If your only light source was one exact frequency of blue light and you shined it on a shirt that was red in sunlight, the shirt would appear black because no light would be reflected. The light from fluorescent types of lights do not contain all the frequencies of sunlight and so clothes inside a store may appear to be a slightly different color than when you get them home.
Continuous and Line Spectra
Electric light bulbs contain a very thin wire in them that emits light when heated. The wire is called a filament. The particular wire used in light bulbs is made of tungsten. A wire made of any metal would emit light under these circumstances but tungsten was chosen because the light it emits contains virtually every frequency and therefore, the light emitted by tungsten appears white. A wire made of some other element would emit light of some color that was not convenient for our uses. Every element emits light when energized by heating or passing electric current through it. Elements in solid form begin to glow when they are heated sufficiently and elements in gaseous form emit light when electricity passes through them. This is the source of light emitted by neon signs and is also the source of light in a fire.

Each Element Has a Unique Spectrum
The light frequencies emitted by atoms are mixed together by our eyes so that we see a blended color. Several physicists, including Angstrom in 1868 and Balmer in 1875, passed the light from energized atoms through glass prisms in such a way that the light was spread out so they could see the individual frequencies that made up the light. The emission spectrum (or atomic spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity.

When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. But when the color is spread out, we see that the hydrogen spectrum is composed of four individual frequencies. The pink color of the tube is the result of our eyes blending the four colors. Every atom has its own characteristic spectrum; no two atomic spectra are alike. The image below shows the emission spectrum of iron. Because each element has a unique emission spectrum, elements can be defined using them.
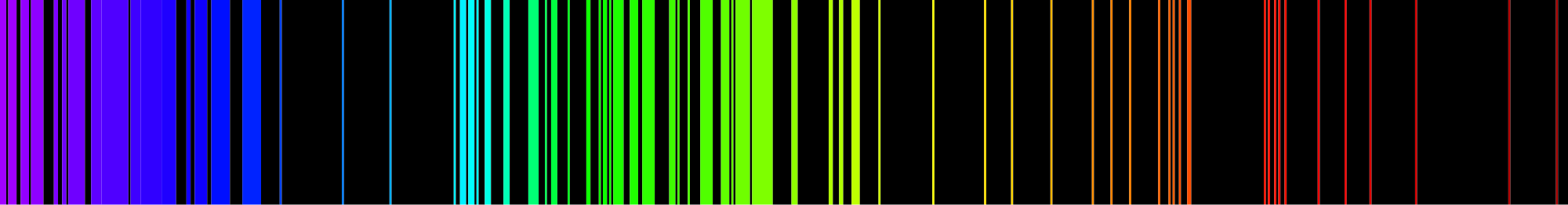
You may have heard or read about scientists discussing what elements are present in the sun or some more distant star, and after hearing that, wondered how scientists could know what elements were present in a place no one has ever been. Scientists determine what elements are present in distant stars by analyzing the light that comes from stars and finding the atomic spectrum of elements in that light. If the exact four lines that compose hydrogen's atomic spectrum are present in the light emitted from the star, that element contains hydrogen.
Bohr's Explanation of Line Spectra
In 1913, the Danish physicist Niels Bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to produce atomic spectra. Understanding Bohr's model requires some knowledge of electromagnetic radiation (or light). Bohr's key idea in his model of the atom is that electrons occupy definite orbitals that require the electron to have a specific amount of energy. In order for an electron to be in the electron cloud of an atom, it must be in

one of the allowable orbitals and it must have the precise energy required for that orbit. Orbits closer to the nucleus would require smaller amounts of energy for an electron and orbits farther from the nucleus would require the electrons to have a greater amount of energy. The possible orbits are known as energy levels (n). One of the weaknesses of Bohr's model was that he could not offer a reason why only certain energy levels or orbits were allowed.
Figure \(\PageIndex{5}\): Niels Bohr with Albert Einstein at Paul Ehrenfest's home in Leiden (December 1925).

Bohr hypothesized that the only way electrons could gain or lose energy would be to move from one energy level to another, thus gaining or losing precise amounts of energy. The energy levels are quantized, meaning that only specific amounts are possible. It would be like a ladder that had rungs only at certain heights. The only way you can be on that ladder is to be on one of the rungs and the only way you could move up or down would be to move to one of the other rungs. Suppose we had such a ladder with 10 rungs. Other rules for the ladder are that only one person can be on a rung in normal state and the ladder occupants must be on the lowest rung available. If the ladder had five people on it, they would be on the lowest five rungs. In this situation, no person could move down because all the lower rungs are full. Bohr worked out rules for the maximum number of electrons that could be in each energy level in his model and required that an atom in its normal state (ground state) had all electrons in the lowest energy levels available. Under these circumstances, no electron could lose energy because no electron could move down to a lower energy level. In this way, Bohr's model explained why electrons circling the nucleus did not emit energy and spiral into the nucleus.
Figure \(\PageIndex{6}\) The energy levels (n= 1,2,3...) of the electrons can be viewed as rungs on a ladder.
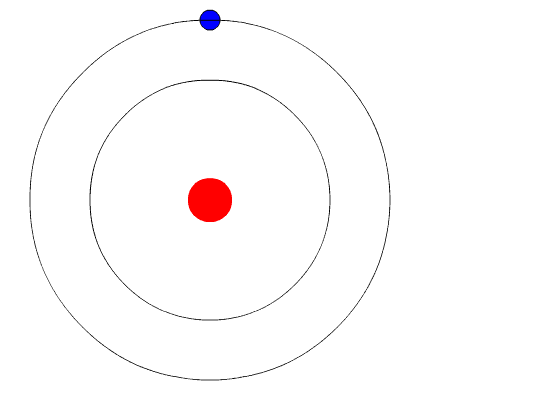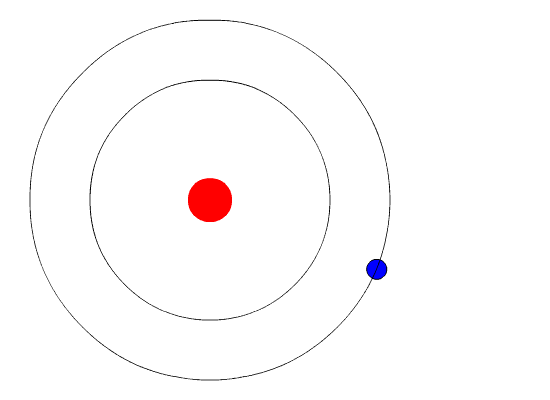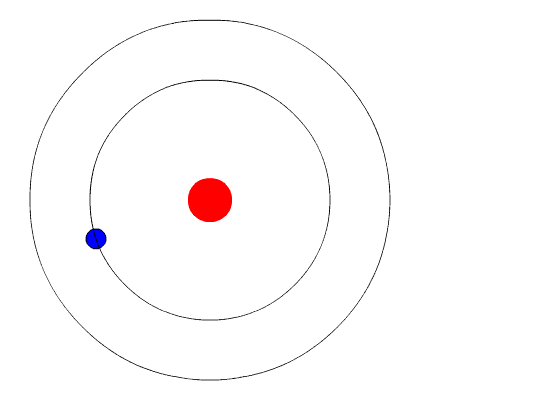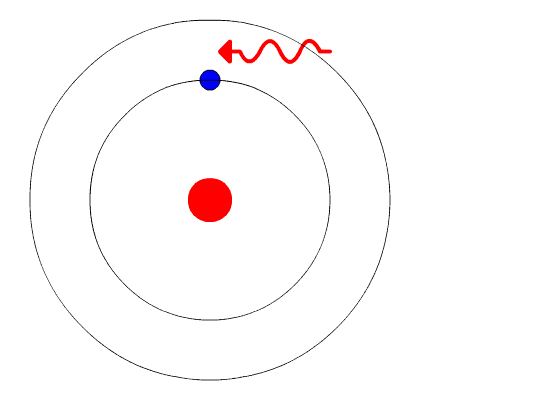
The evidence used to support Bohr's model came from the atomic spectra. He suggested that an atomic spectrum is made by the electrons in an atom moving energy levels.
Ground States and Excited States
The electrons typically have the lowest energy possible, called the ground state. If the electrons are given energy (through heat, electricity, light, etc.) the electrons in an atom could absorb energy by jumping to a higher energy level, or excited state. The electrons then give off the energy in the form of a piece of light, called a photon, they had absorbed to fall back to a lower energy level. The energy emitted by electrons dropping back to lower energy levels would always be precise amounts of energy because the differences in energy levels were precise. This explains why you see specific lines of light when looking at an atomic spectrum - each line of light matches a specific "step down" that an electron can take in that atom. This also explains why each element produces a different atomic spectrum. Because each element has different acceptable energy levels for their electrons, the possible steps each element's electrons can take differ from all other elements.
Based on the wavelengths of the spectral lines, Bohr was able to calculate the energies that the hydrogen electron would have in each of its allowed energy levels. He then mathematically showed which energy level transitions correspond to the spectral lines in the atomic emission spectrum (see below).
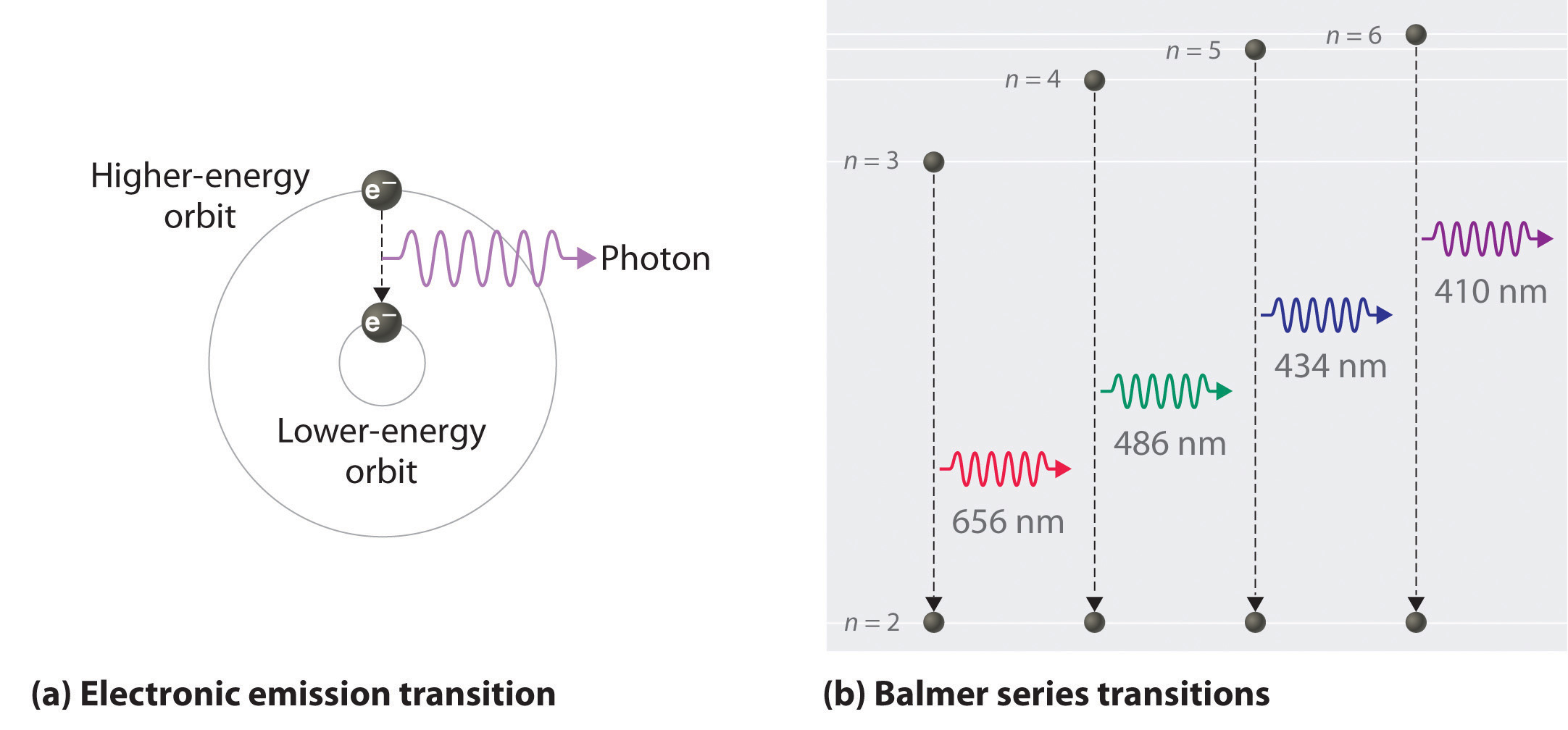
He found that the four visible spectral lines corresponded to transitions from higher energy levels down to the second energy level \(\left( n=2 \right)\). This is called the Balmer series (Figure \(\PageIndex{8}\) ). Transitions ending in the ground state \(\left( n=1 \right)\) are called the Lyman series, but the energies released are so large that the spectral lines are all in the ultraviolet region of the spectrum. The transitions called the Paschen series and the Brackett series both result in spectral lines in the infrared region because the energies are too small.
Bohr's model was a tremendous success in explaining the spectrum of the hydrogen atom. Unfortunately, when the mathematics of the model was applied to atoms with more than one electron, it was not able to correctly predict the frequencies of the spectral lines. While Bohr's model represented a great advancement in the atomic model and the concept of electron transitions between energy levels is valid, improvements were needed in order to fully understand all atoms and their chemical behavior.
Different metal electrons emit different wavelengths of light to return to their respective ground states, so the flame colors are varied. These flames can be used to produce atomic emmision spectra of the elements combusted. Using known values of emission spectra, one can perform a flame test on un unknown substance, gather an emmision spectrum from it, and determine which elements are in the unknown substance.
For example, in the case of copper ion, there are multiple different "paths" that the excited electrons can follow to emit photon of certain discrete energy. This produces multiple spectra lines because each discrete energy level difference will yield a specific wavelength of light, which determines the color.


Building Atoms: Main Shells
An electron shell is the outside part of an atom around the atomic nucleus. It is a group of atomic orbitals with the same value of the principal quantum number \(n\). Electron shells have one or more electron subshells, or sublevels. The name for electron shells comes from the Bohr model, in which groups of electrons were believed to go around the nucleus at certain distances, so that their orbits formed "shells".
An electron shell may be thought of as an orbit followed by electrons around an atom nucleus. Because each shell can contain only a fixed number of electrons, each shell is associated with a particular range of electron energy, and thus each shell must fill completely before electrons can be added to an outer shell. The electrons in the outermost shell determine the chemical properties of the atom (see Valence shell). For an explanation of why electrons exist in these shells see electron configuration.
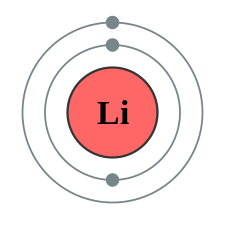
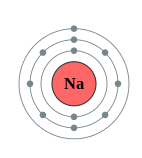
Figure \(\PageIndex{10}\) A shell diagram of lithium (left) and Sodium (right)
The electron shells are labeled K, L, M, N, O, P, and Q; or 1, 2, 3, 4, 5, 6, and 7; going from innermost shell outwards. Electrons in outer shells have higher average energy and travel farther from the nucleus than those in inner shells. This makes them more important in determining how the atom reacts chemically and behaves as a conductor, because the pull of the atom's nucleus upon them is weaker and more easily broken. In this way, a given element's reactivity is highly dependent upon its electronic configuration.
Video \(\PageIndex{1}\) How to draw the shell model for sulfur.
Note: The number of electrons that can occupy each energy level are 2 (first level), 8 (2nd level), 18 (3rd level), and 32 (4th level) based on the formula: # of electrons = 2(n)2, wherein n = principle energy level.
Table \(\PageIndex{1}\) shows the number of electrons that fill each shell for neutral atoms of several elements. As mentioned earlier, the innermost shell (corresponding to lowest energy) is filled first and only a fixed number of electrons is allowed in each shell. The only electron in hydrogen (Z=1) goes to the first shell. In lithium atom (Z=3), the two electrons fill the first shell, and the third electron goes to the second shell. An argon atom (Z=18) has 18 electrons. The 10 electrons fill the first and second shells, and the remaining 8 electrons go to the third shell. The electron configuration for elements pass argon are covered in more detail in section 3.7.
|
Element Symbol |
Atomic Number* (Z) |
First Shell n=1 (2 electrons allowed) |
Second Shell n=2 (8 electrons allowed) |
Third Shell n=3 (18 electrons allowed) |
|---|---|---|---|---|
| H | 1 | 1 | ||
| He | 2 | 2 | ||
| C | 6 | 2 | 4 | |
| N | 7 | 2 | 5 | |
| Na | 11 | 2 | 8 | 1 |
| Mg | 12 | 2 | 8 | 2 |
| Cl | 17 | 2 | 8 | 7 |
| Ar | 18 | 2 | 8 | 8 |
Note:* In a neutral atom he number of protons is equal to the number of electrons.
Summary
- Electromagnetic radiation has a wide spectrum, including gamma rays, X-rays, UV rays, visible light, IR radiation, microwaves, and radio waves.
- The different colors of light differ in their frequencies (or wavelengths).
- Bohr's model suggests each atom has a set of unchangeable energy levels and electrons in the electron cloud of that atom must be in one of those energy levels.
- Bohr's model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source, jumping up to a higher energy level, then immediately dropping back to a lower energy level and emitting the energy different between the two energy levels.
- The existence of the atomic spectra is support for Bohr's model of the atom.
- Bohr's model was only successful in calculating energy levels for the hydrogen atom.
- The shell model is a good representation of electron arrangement only for elements 1-18.
Contributors and Attributions
- Connections (John Hutchinson)
- Wikipedia
Henry Agnew (UC Davis)

