3.5: The Atomic Nucleus
- Page ID
- 152151
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- List the properties of the three main subatomic particles.
- Define atomic mass unit (amu).
- Define atomic number and mass number.
- Define isotopes.
- Determine the number of protons, neutrons, and electrons in an atom with a given mass number.
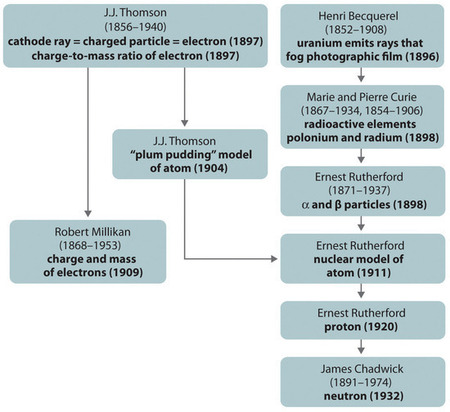
Figure \(\PageIndex{1}\) A summary of the historical development of models of the components and structure of the atom. The dates in parentheses are the years in which the key experiments were performed. (CC BY-SA-NC).
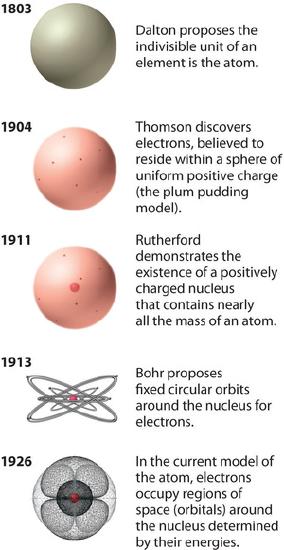 Rutherford's model of the atom is essentially the same as the modern model, except that it is now known that electrons are not uniformly distributed throughout an atom’s volume. Instead, they are distributed according to a set of principles described by Quantum Mechanics. Figure \(\PageIndex{2}\) shows how the model of the atom has evolved over time from the indivisible unit of Dalton to the modern view taught today.
Rutherford's model of the atom is essentially the same as the modern model, except that it is now known that electrons are not uniformly distributed throughout an atom’s volume. Instead, they are distributed according to a set of principles described by Quantum Mechanics. Figure \(\PageIndex{2}\) shows how the model of the atom has evolved over time from the indivisible unit of Dalton to the modern view taught today.Figure \(\PageIndex{2}\)The Evolution of Atomic Theory, as Illustrated by Models of the Oxygen Atom. Bohr’s model and the current model are described in Chapter 6, "The Structure of Atoms." Image used with Permission (CC BY-SA-NC).
The nucleus (plural, nuclei) is a positively charged region at the center of the atom. It consists of two types of subatomic particles packed tightly together. The particles are protons, which have a positive electric charge, and neutrons, which are neutral in electric charge. Outside of the nucleus, an atom is mostly empty space, with orbiting negative particles called electrons whizzing through it. Figure \(\PageIndex{3}\) below shows these parts of the atom.
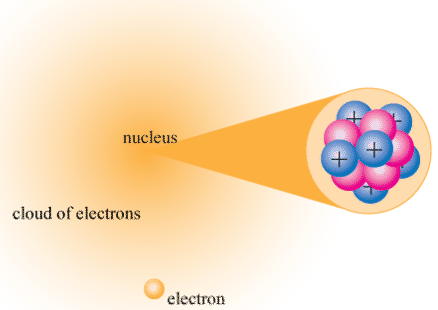
The nucleus of the atom is extremely small. Its radius is only about 1/100,000 of the total radius of the atom. If an atom were the size of a football stadium, the nucleus would be about the size of a pea! Electrons have virtually no mass, but protons and neutrons have a lot of mass for their size. As a result, the nucleus has virtually all the mass of an atom. Given its great mass and tiny size, the nucleus is very dense. If an object the size of a penny had the same density as the nucleus of an atom, its mass would be greater than 30 million tons!
Particles with opposite electric charges attract each other. This explains why negative electrons orbit the positive nucleus. Particles with the same electric charge repel each other. This means that the positive protons in the nucleus push apart from one another. So why doesn't the nucleus fly apart? An even stronger force - called the strong nuclear force - holds protons and neutrons together in the nucleus.
| Particle | Symbol | Mass (amu) | Relative Mass (proton = 1) | Relative Charge | Location |
|---|---|---|---|---|---|
| proton | p+ | 1 | 1 | +1 | inside the nucleus |
| electron | e− | 5.45× 10−4 | 0.00055 | −1 | outside nucleus |
| neutron | n0 | 1 | 1 | 0 | inside the nucleus |
1 amu = 1.6605 × 10−24 g = 1.6605 × 10−27 kg
Atomic Number
Negative and positive charges of equal magnitude cancel each other out. This means that the negative charge on an electron perfectly balances the positive charge on the proton. In other words, a neutral atom must have exactly one electron for every proton. If a neutral atom has 1 proton, it must have 1 electron. If a neutral atom has 2 protons, it must have 2 electrons. If a neutral atom has 10 protons, it must have 10 electrons. You get the idea. In order to be neutral, an atom must have the same number of electrons and protons.
Scientists distinguish between different elements by counting the number of protons in the nucleus (Table \(\PageIndex{2}\)). If an atom has only one proton, we know it's a hydrogen atom. An atom with two protons is always a helium atom. If scientists count four protons in an atom, they know it's a beryllium atom. An atom with three protons is a lithium atom, an atom with five protons is a boron atom, an atom with six protons is a carbon atom . . . the list goes on.
Since an atom of one element can be distinguished from an atom of another element by the number of protons in its nucleus, scientists are always interested in this number, and how this number differs between different elements. The number of protons in an atom is called its atomic number (\(Z\)). This number is very important because it is unique for atoms of a given element. All atoms of an element have the same number of protons, and every element has a different number of protons in its atoms. For example, all helium atoms have two protons, and no other elements have atoms with two protons.
| Name | Protons | Neutrons | Electrons | Atomic Number (Z) | Mass Number (A) |
|---|---|---|---|---|---|
| Hydrogen | 1 | 0 | 1 | 1 | 1 |
| Helium | 2 | 2 | 2 | 2 | 4 |
| Lithium | 3 | 4 | 3 | 3 | 7 |
| Beryllium | 4 | 5 | 4 | 4 | 9 |
| Boron | 5 | 6 | 5 | 5 | 11 |
| Carbon | 6 | 6 | 6 | 6 | 12 |
Of course, since neutral atoms have to have one electron for every proton, an element's atomic number also tells you how many electrons are in a neutral atom of that element. For example, hydrogen has an atomic number of 1. This means that an atom of hydrogen has one proton, and, if it's neutral, one electron as well. Gold, on the other hand, has an atomic number of 79, which means that an atom of gold has 79 protons, and, if it's neutral, 79 electrons as well.
Atoms are neutral in electrical charge because they have the same number of negative electrons as positive protons (Table \(\PageIndex{2}\)). Therefore, the atomic number of an atom also tells you how many electrons the atom has. This, in turn, determines many of the atom's chemical properties.
Mass Number
The mass number (\(A\)) of an atom is the total number of protons and neutrons in its nucleus. The mass of the atom is a unit called the atomic mass unit \(\left( \text{amu} \right)\). One atomic mass unit is the mass of a proton, or about \(1.67 \times 10^{-27}\) kilograms, which is an extremely small mass. A neutron has just a tiny bit more mass than a proton, but its mass is often assumed to be one atomic mass unit as well. Because electrons have virtually no mass, just about all the mass of an atom is in its protons and neutrons. Therefore, the total number of protons and neutrons in an atom determines its mass in atomic mass units (Table \(\PageIndex{2}\)).
Consider helium again. Most helium atoms have two neutrons in addition to two protons. Therefore the mass of most helium atoms is 4 atomic mass units (\(2 \: \text{amu}\) for the protons + \(2 \: \text{amu}\) for the neutrons). However, some helium atoms have more or less than two neutrons. Atoms with the same number of protons but different numbers of neutrons are called isotopes. Because the number of neutrons can vary for a given element, the mass numbers of different atoms of an element may also vary. For example, some helium atoms have three neutrons instead of two (these are called isotopes and are discussed in detail later on)
Why do you think that the "mass number" includes protons and neutrons, but not electrons? You know that most of the mass of an atom is concentrated in its nucleus. The mass of an atom depends on the number of protons and neutrons. You have already learned that the mass of an electron is very, very small compared to the mass of either a proton or a neutron (like the mass of a penny compared to the mass of a bowling ball). Counting the number of protons and neutrons tells scientists about the total mass of an atom. An atom's mass number is very easy to calculate provided you know the number of protons and neutrons in an atom. The mass number of a carbon atom with 6 protons and 7 neutrons is calculated and shown as follows:
\[\text{mass number} \: A = \left( \text{number of protons} \right) + \left( \text{number of neutrons} \right) \nonumber \]
\[\text{mass number} = \text{6} + \text{6} = \text{12} \nonumber \]

What is the mass number of an atom of helium that contains 2 neutrons?
Solution
\(\left( \text{number of protons} \right) = 2\) (Remember that an atom of helium always has 2 protons.)
\(\left( \text{number of neutrons} \right) = 2\)
\(\text{mass number} = \left( \text{number of protons} \right) + \left( \text{number of neutrons} \right)\)
\(\text{mass number} = 2 + 2 = 4\)
Isotopes
All atoms of the same element have the same number of protons, but some may have different numbers of neutrons. For example, all carbon atoms have six protons, and most have six neutrons as well. But some carbon atoms have seven or eight neutrons instead of the usual six. Atoms of the same element that differ in their numbers of neutrons are called isotopes. Many isotopes occur naturally. Usually one or two isotopes of an element are the most stable and common. Different isotopes of an element generally have the same physical and chemical properties. That's because they have the same numbers of protons and electrons.
An Example: Hydrogen Isotopes
Hydrogen is an example of an element that has isotopes. Three isotopes of hydrogen are modeled in Figure \(\PageIndex{4}\). Most hydrogen atoms have just one proton and one electron and lack a neutron. These atoms are just called hydrogen. Some hydrogen atoms have one neutron as well. These atoms are the isotope named deuterium. Other hydrogen atoms have two neutrons. These atoms are the isotope named tritium.
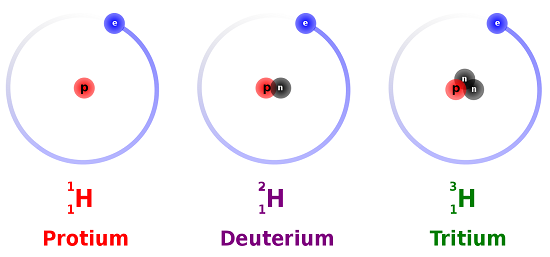
For most elements other than hydrogen, isotopes are named for their mass number. For example, carbon atoms with the usual 6 neutrons have a mass number of 12 (6 protons + 6 neutrons = 12), so they are called carbon-12. Carbon atoms with 7 neutrons have atomic mass of 13 (6 protons + 7 neutrons = 13). These atoms are the isotope called carbon-13.
- What is the atomic number and the mass number of an isotope of lithium containing 3 neutrons.
- What is the atomic number and the mass number of an isotope of lithium containing 4 neutrons?
Solution
A lithium atom contains 3 protons in its nucleus irrespective of the number of neutrons or electrons.
a.
\[ \begin{align}\text{atomic number} = \left( \text{number of protons} \right) &= 3 \nonumber \\ \left( \text{number of neutrons} \right) &= 3 \nonumber\end{align} \nonumber \]
\[ \begin{align} \text{mass number} & = \left( \text{number of protons} \right) + \left( \text{number of neutrons} \right) \nonumber\\ \text{mass number} & = 3 + 3 \nonumber\\ &= 6 \nonumber \end{align}\nonumber \]
b.
\[ \begin{align}\text{atomic number} = \left( \text{number of protons} \right) &= 3 \nonumber\\ \left( \text{number of neutrons} \right) & = 4\nonumber\end{align}\nonumber \]
\[ \begin{align}\text{mass number} & = \left( \text{number of protons} \right) + \left( \text{number of neutrons} \right)\nonumber \\ \text{mass number} & = 3 + 4\nonumber \\ &= 7 \nonumber \end{align}\nonumber \]
Notice that because the lithium atom always has 3 protons, the atomic number for lithium is always 3. The mass number, however, is 6 in the isotope with 3 neutrons, and 7 in the isotope with 4 neutrons. In nature, only certain isotopes exist. For instance, lithium exists as an isotope with 3 neutrons, and as an isotope with 4 neutrons, but it doesn't exist as an isotope with 2 neutrons or as an isotope with 5 neutrons.
The number of protons in the nucleus of a tin atom is 50, while the number of neutrons in the nucleus is 68. What are the atomic number and the mass number of this isotope?
- Answer
-
Atomic number = 50, mass number = 118
Symbols for Isotopes
There are two main ways in which scientists frequently show the mass number of an atom they are interested in. It is important to note that the mass number is not given on the periodic table. These two ways include writing a nuclear symbol or by giving the name of the element with the mass number written.
To write a nuclear symbol, the mass number is placed at the upper left (superscript) of the chemical symbol and the atomic number is placed at the lower left (subscript) of the symbol. The complete nuclear symbol for helium-4 is drawn below:

The following nuclear symbols are for a nickel nucleus with 31 neutrons and a uranium nucleus with 146 neutrons.
\[\ce{^{59}_{28}Ni} \nonumber \]
\[ \ce{ ^{238}_{92}U} \nonumber \]
In the nickel nucleus represented above, the atomic number 28 indicates the nucleus contains 28 protons, and therefore, it must contain 31 neutrons in order to have a mass number of 59. The uranium nucleus has 92 protons as do all uranium nuclei and this particular uranium nucleus has 146 neutrons.
Another way of representing isotopes is by adding a hyphen and the mass number to the chemical name or symbol. Thus the two nuclei would be Nickel-59 or Ni-59 and Uranium-238 or U-238, where 59 and 238 are the mass numbers of the two atoms, respectively. Note that the mass numbers (not the number of neutrons) are given to the side of the name.
How many protons, electrons, and neutrons are in an atom of \(^{40}_{19}\ce{K}\)?
Solution
\[\text{atomic number} = \left( \text{number of protons} \right) = 19 \nonumber \]
For all atoms with no charge, the number of electrons is equal to the number of protons.
\[\text{number of electrons} = 19 \nonumber \]
The mass number, 40 is the sum of the protons and the neutrons.
To find the number of neutrons, subtract the number of protons from the mass number.
\[\text{number of neutrons} = 40 - 19 = 21. \nonumber \]
How many protons, electrons, and neutrons are in an atom of zinc-65?
Solution
\[\text{number of protons} = 30 \nonumber \]
For all atoms with no charge, the number of electrons is equal to the number of protons.
\[\text{number of electrons} = 30 \nonumber \]
The mass number, 65 is the sum of the protons and the neutrons.
To find the number of neutrons, subtract the number of protons from the mass number.
\[\text{number of neutrons} = 65 - 30 = 35 \nonumber \]
How many protons, electrons, and neutrons are in each atom?
- \(^{60}_{27}\ce{Co}\)
- Na-24
- \(^{45}_{20}\ce{Ca}\)
- Sr-90
- Answer a:
- 27 protons, 27 electrons, 33 neutrons
- Answer b:
- 11 protons, 11 electrons, 13 neutrons
- Answer c:
- 20 protons, 20 electrons, 25 neutrons
- Answer d:
- 38 protons, 38 electrons, 52 neutrons
| Element | Symbol | Atomic Number | Number of Protons | Number of Neutrons | Mass (amu) | % Natural Abundance |
|---|---|---|---|---|---|---|
| hydrogen | \(\ce{^1_1H}\) (protium) |
1 | 1 | 0 | 1.0078 | 99.989 |
| \(\ce{^2_1H}\) (deuterium) |
1 | 1 | 1 | 2.0141 | 0.0115 | |
| \(\ce{^3_1H}\) (tritium) |
1 | 1 | 2 | 3.01605 | — (trace) | |
| helium | \(\ce{^3_2He}\) | 2 | 2 | 1 | 3.01603 | 0.00013 |
| \(\ce{^4_2He}\) | 2 | 2 | 2 | 4.0026 | 100 | |
| lithium | \(\ce{^6_3Li}\) | 3 | 3 | 3 | 6.0151 | 7.59 |
| \(\ce{^7_3Li}\) | 3 | 3 | 4 | 7.0160 | 92.41 | |
| beryllium | \(\ce{^9_4Be}\) | 4 | 4 | 5 | 9.0122 | 100 |
| boron | \(\ce{^{10}_5B}\) | 5 | 5 | 5 | 10.0129 | 19.9 |
| \(\ce{^{11}_5B}\) | 5 | 5 | 6 | 11.0093 | 80.1 | |
| carbon | \(\ce{^{12}_6C}\) | 6 | 6 | 6 | 12.0000 | 98.89 |
| \(\ce{^{13}_6C}\) | 6 | 6 | 7 | 13.0034 | 1.11 | |
| \(\ce{^{14}_6C}\) | 6 | 6 | 8 | 14.0032 | — (trace) |
Summary
- The atom consists of discrete particles that govern its chemical and physical behavior.
- Each atom of an element contains the same number of protons, which is the atomic number (Z).
- Neutral atoms have the same number of electrons and protons.
- Atoms of an element that contain different numbers of neutrons are called isotopes.
- Each isotope of a given element has the same atomic number but a different mass number (A), which is the sum of the numbers of protons and neutrons.
- The relative masses of atoms are reported using the atomic mass unit (amu) which is defined as one-twelfth of the mass of one atom of carbon-12, with 6 protons, 6 neutrons, and 6 electrons. The nuclear model of the atom consists of a small and dense positively charged interior surrounded by a cloud of electrons.
Contributors and Attributions
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).
- TextMap: Chemistry-The Central Science (Brown et al.)
- TextMap: Fundamentals of General, Organicn, and Biological Chemistry (McMurry et al.)
Henry Agnew (UC Davis)


\[\begin{alignat*}{3} &[x \mapsto s]x &&= s && \\ &[x \mapsto s]y &&= y \qquad &&\text{als } y\neq x \\ &[x \mapsto s](\lambda(y)t_1) &&= \lambda(y)[x \mapsto s]t_1 \qquad &&\text{als } y \neq x \text{ en } y \not \in FV(s) \\ &[x \mapsto s](t_1 \; t_2)&&= ([x \mapsto s]t_1)\;([x \mapsto s]t_2) && \end{alignat*} \nonumber \]