Chemistry of Vanadium
- Page ID
- 3717
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Vanadium takes its name from the Scandinavian goddess Vanadis and was discovered in 1801 by Andrés Manuel del Rio. It was isolated in 1867 by Henry Roscoe as a silvery-white metal that is somewhat heavier than aluminum but lighter than iron. It has excellent corrosion resistance at room temperature.
The history of its discovery is an interesting tale. del Rio sent his brown ore samples, containing what he thought was a new element to Paris for analysis and confirmation, along with a brief explanation that was ambiguous. The complete analysis and description of his work were lost in a shipwreck so the Paris lab saw nothing but brown powder and a brief confusing note. A second sample sent to Berlin was mislabeled lead chromate when it arrived. del Rio gave up, losing confidence in his discovery. The element was rediscovered in 1867 by Nils Sefstrôm.
Vanadium has an unusually large number of stable oxidation states (+2, +3, +4, +5)each of which is characterized by a unique color in solution. The metal is used as an alloying agent for steel. It combines with nearly all non-metals in compounds.
Vanadium(V) oxide as a Catalyst
During the Contact Process for manufacturing sulfuric acid, sulfur dioxide has to be converted into sulfur trioxide, which is done by passing sulfur dioxide and oxygen over a solid vanadium(V) oxide catalyst.
\[ SO_2 + \dfrac{1}{2}O_2 \ce{->[V_2O_5]} SO_3 \nonumber \]
This is a good example of the ability of transition metals and their compounds to act as catalysts because of their ability to change their oxidation state (oxidation number). The sulfur dioxide is oxidized to sulfur trioxide by the vanadium(V) oxide. In the process, the vanadium(V) oxide is reduced to vanadium(IV) oxide.
\[ SO_2 + V_2O_5 \rightarrow SO_3 + V_2O_4 \nonumber \]
The vanadium(IV) oxide is then re-oxidized by the oxygen.
\[ V_2O_4 + \dfrac{1}{2} O_2 \rightarrow V_2O_5 \nonumber \]
Although the catalyst has been temporarily changed during the reaction, at the end it is chemically the same as it started.
Vanadium's oxidation states
Vanadium has oxidation states in its compounds of +5, +4, +3 and +2. This section looks at ways of changing between them. It starts with a bit of description, and then goes on to look at the reactions in terms of standard redox potentials (standard electrode potentials).

Reducing vanadium(V) in stages to vanadium(II)
The usual source of vanadium in the +5 oxidation state is ammonium metavanadate, NH4VO3. This isn't very soluble in water and is usually first dissolved in sodium hydroxide solution. The solution can be reduced using zinc and an acid - either hydrochloric acid or sulfuric acid, usually using moderately concentrated acid. The exact vanadium ion present in the solution is very complicated, and varies with the pH of the solution. The reaction is done under acidic conditions when the main ion present is VO2+ - called the dioxovanadium(V) ion.
If you do the reaction in a small flask, it is normally stoppered with some cotton wool. This allows hydrogen (produced from a side reaction between the zinc and acid) to escape. At the same time it stops much air from entering. This prevents re-oxidation of the lower oxidation states of vanadium (particularly the +2 state) by oxygen in the air. The reaction is usually warmed so that the changes happen in a reasonable time. The reduction is shown in two stages. Some individual important colors are shown, but the process is one continuous change from start to finish.
The reduction from +5 to +4

It is important to notice that the green color you see isn't actually another oxidation state. it is just a mixture of the original yellow of the +5 state and the blue of the +4. Be very careful with the formulae of the two vanadium ions - they are very easy to confuse!
The reduction from +4 to +2. The color changes just continue.
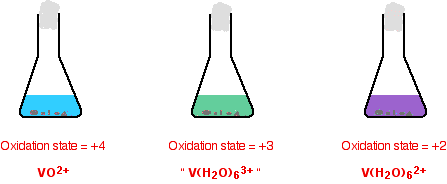
The reason for the inverted commas around the vanadium(III) ion is that this is almost certainly a simplification. The exact nature of the complex ion will depend on which acid you use in the reduction process. The simplification is probably reasonable at this level.
Re-oxidation of the vanadium(II)
The vanadium(II) ion is very easily oxidized. If you remove the cotton wool from the flask and pour some solution into a test tube, it turns green because of its contact with oxygen in the air. It is oxidized back to vanadium(III). If it is allowed to stand for a long time, the solution eventually turns blue as the air oxidizes it back to the vanadium(IV) state - VO2+ ions. Adding nitric acid (a reasonably powerful oxidizing agent) to the original vanadium(II) solution also produces blue VO2+ ions. The vanadium(II) is again oxidized back to vanadium(IV).
Using Zinc as the reducing agent
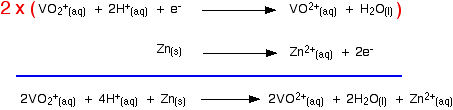
Let's look at the first stage of the reduction - from VO2+ to VO2+. The redox potential for the vanadium half-reaction is given by:
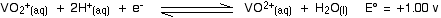
The corresponding equilibrium for the zinc is:
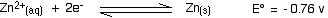
The simple principle is that if you couple two of these half-reactions together, the one with the more positive E° value will move to the right; the one with the more negative (or less positive) E° moves to the left. If you mix together zinc and VO2+ ions in the presence of acid to provide the H+ ions:
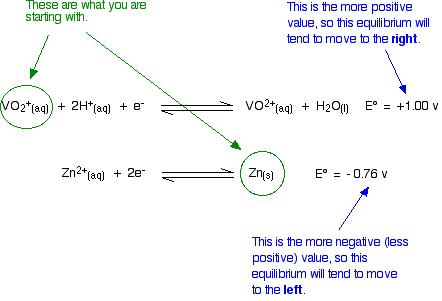
That converts the two equilibria into two one-way reactions. You can write these down and combine them to give the ionic equation for the reaction if you want to.
The other stages of the reaction
Here are the E° values for all the steps of the reduction from vanadium(V) to vanadium(II):
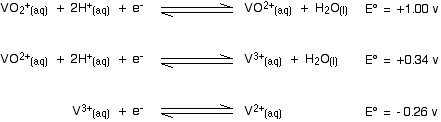
. . . and here is the zinc value again:
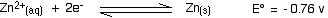
Remember that for the vanadium reactions to move to the right (which is what we want), their E° values must be more positive than whatever you are reacting them with. In other words, for the reactions to work, zinc must always have the more negative value - and that's the case. Zinc can reduce the vanadium through each of these steps to give the vanadium(II) ion.
Using other reducing agents
Suppose you replaced zinc as the reducing agent by tin. How far would the set of reductions go this time?
Here are the E° values again:
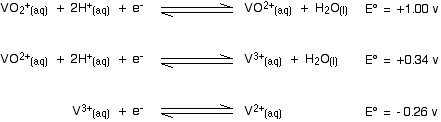
. . . and here is the tin value:
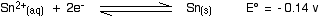
For each reduction to happen, the vanadium reaction has to have the more positive E° value because we want it to go to the right. That means that the tin must have the more negative value.
- In the first vanadium equation (from +5 to +4), the tin value is more negative. That works OK.
- In the second vanadium equation (from +4 to +3), the tin value is again the more negative. That works as well.
- But in the final vanadium reaction (from +3 to +2), tin no longer has the more negative E° value. Tin won't reduce vanadium(III) to vanadium(II).
Re-oxidation of the vanadium(II)
The vanadium(II) oxidation state is easily oxidized back to vanadium(III) - or even higher.
Oxidation by hydrogen ions
You will remember that the original reduction we talked about was carried out using zinc and an acid in a flask stoppered with a piece of cotton wool to keep the air out. Air will rapidly oxidize the vanadium(II) ions - but so also will the hydrogen ions present in the solution!
The vanadium(II) solution is only stable as long as you keep the air out, and in the presence of the zinc. The zinc is necessary to keep the vanadium reduced. What happens if the zinc isn't there? Look at these E° values:
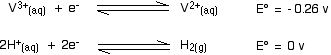
The reaction with the more negative E° value goes to the left; the reaction with the more positive (or less negative) one to the right. That means that the vanadium(II) ions will be oxidized to vanadium(III) ions, and the hydrogen ions reduced to hydrogen.
Will the oxidation go any further - for example, to the vanadium(IV) state? Have a look at the E° values and decide:
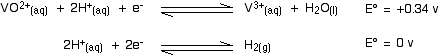
In order for the vanadium equilibrium to move to the left, it would have to have the more negative E° value. It hasn't got the more negative E° value and so the reaction does not happen.
Oxidation by nitric acid
In a similar sort of way, you can work out how far nitric acid will oxidize the vanadium(II). Here's the first step:
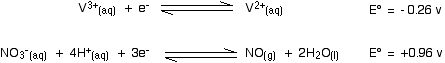
The vanadium reaction has the more negative E° value and so will move to the left; the nitric acid reaction moves to the right.
Nitric acid will oxidize vanadium(II) to vanadium(III). The second stage involves these E° values:
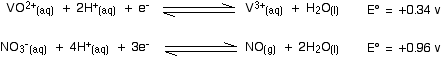
The nitric acid again has the more positive E° value and so moves to the right. The more negative (less positive) vanadium reaction moves to the left. Nitric acid will certainly oxidize vanadium(III) to vanadium(IV). Will it go all the way to vanadium(V)?
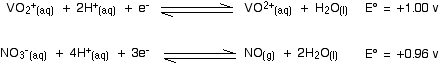
No, it won't! For the vanadium reaction to move to the left to form the dioxovanadium(V) ion, it would have to have the more negative (less positive) E° value. It hasn't got a less positive value, and so the reaction does not happen.
You can work out the effect of any other oxidizing agent on the lower oxidation states of vanadium in exactly the same way. But don't assume that because the E° values show that a reaction is possible, it will necessarily happen.
Contributors and Attributions
Jim Clark (Chemguide.co.uk)
Stephen R. Marsden


